Question
Question: Which of the following compound exist -...
Which of the following compound exist -
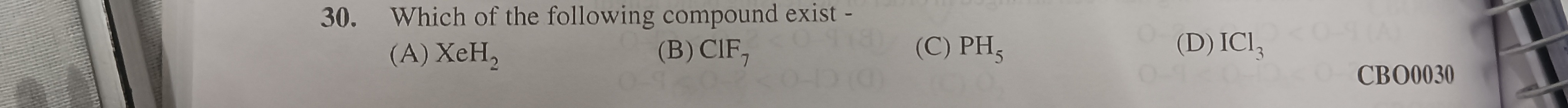
A
XeH₂
B
CIF₇
C
PH₅
D
ICl₃
Answer
D
Explanation
Solution
The existence of a compound depends on its thermodynamic stability and kinetic feasibility.
- XeH₂ is unstable due to the low electronegativity of hydrogen and the general inertness of noble gases towards hydrogen.
- ClF₇ does not exist due to the small size of chlorine and high steric hindrance from seven fluorine atoms. The maximum stable fluoride of chlorine is ClF₅.
- PH₅ does not exist because hydrogen is not electronegative enough to stabilize the +5 oxidation state of phosphorus in a hydride.
- ICl₃ is a known and stable interhalogen compound, where iodine expands its octet.
