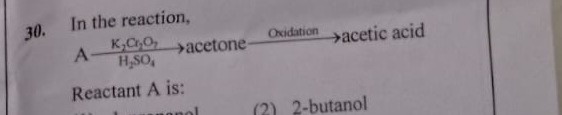
Question
Question: In the reaction, A$\frac{K_2Cr_2O_7}{H_2SO_4}$acetone$\xrightarrow{Oxidation}$acetic acid Reactant ...
In the reaction, AH2SO4K2Cr2O7acetoneOxidationacetic acid
Reactant A is:
Answer
Propan-2-ol
Explanation
Solution
Acidified potassium dichromate oxidizes secondary alcohols to ketones. Acetone (CH3COCH3) is a ketone. The secondary alcohol that yields acetone upon oxidation is propan-2-ol (CH3CH(OH)CH3). The subsequent oxidation of acetone to acetic acid confirms the strong oxidizing conditions capable of further cleaving the ketone.
