Question
Question: Which of the following pairs of species have largest difference in spin only magnetic moment?...
Which of the following pairs of species have largest difference in spin only magnetic moment?
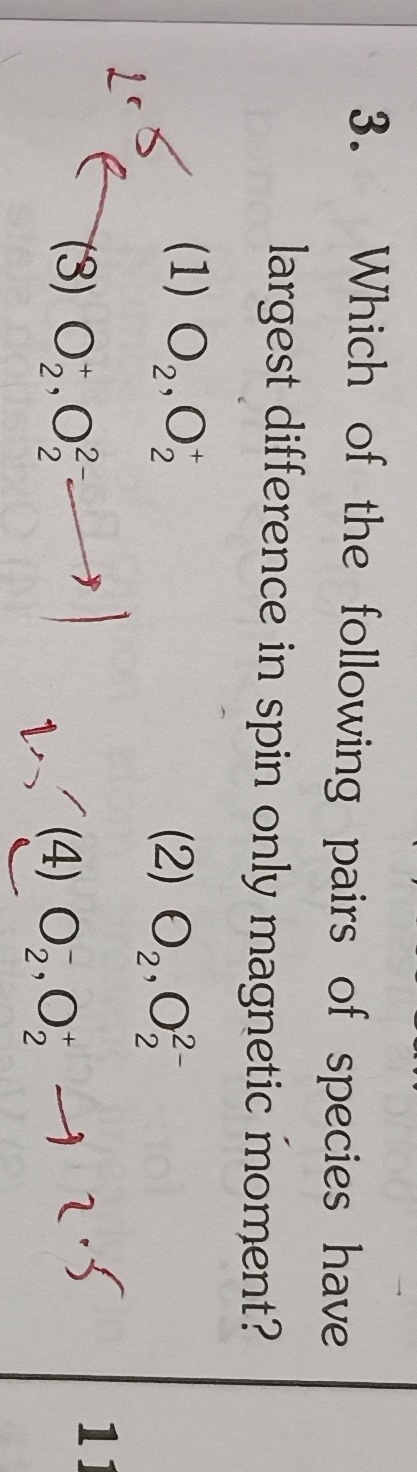
O2,O2+
O2,O2−
O2+,O22−
O2−,O2+
O2+,O22−
Solution
To solve this problem, we need to determine the number of unpaired electrons (n) for each given oxygen species (O2, O2+, O2−, O22−) using Molecular Orbital Theory (MOT). The spin-only magnetic moment (μs) is then calculated using the formula μs=n(n+2) B.M.
The molecular orbital (MO) configuration for O2 and its ions is based on the filling of molecular orbitals formed from atomic orbitals of oxygen atoms. The relevant MOs and their energy order for O2 are: σ1s<σ1s∗<σ2s<σ2s∗<π2p<σ2p<π2p∗<σ2p∗
Let's determine the electronic configuration and the number of unpaired electrons for each species:
-
O2:
- Total electrons = 16 electrons.
- MO configuration: (σ1s)2(σ1s∗)2(σ2s)2(σ2s∗)2(π2p)4(σ2p)2(π2p∗)2
- Number of unpaired electrons (n) = 2.
- Spin-only magnetic moment (μO2) = 2(2+2)=8 B.M.
-
O2+:
- Total electrons = 15 electrons.
- MO configuration: (σ1s)2(σ1s∗)2(σ2s)2(σ2s∗)2(π2p)4(σ2p)2(π2p∗)1
- Number of unpaired electrons (n) = 1.
- Spin-only magnetic moment (μO2+) = 1(1+2)=3 B.M.
-
O2−:
- Total electrons = 17 electrons.
- MO configuration: (σ1s)2(σ1s∗)2(σ2s)2(σ2s∗)2(π2p)4(σ2p)2(π2p∗)3
- Number of unpaired electrons (n) = 1.
- Spin-only magnetic moment (μO2−) = 1(1+2)=3 B.M.
-
O22−:
- Total electrons = 18 electrons.
- MO configuration: (σ1s)2(σ1s∗)2(σ2s)2(σ2s∗)2(π2p)4(σ2p)2(π2p∗)4
- Number of unpaired electrons (n) = 0.
- Spin-only magnetic moment (μO22−) = 0(0+2)=0 B.M.
Now, let's calculate the difference in spin-only magnetic moments for each pair:
-
Pair (1): O2, O2+ Difference = ∣μO2−μO2+∣=∣8−3∣ B.M. ≈1.096 B.M.
-
Pair (2): O2, O2− Difference = ∣μO2−μO2−∣=∣8−3∣ B.M. ≈1.096 B.M.
-
Pair (3): O2+, O22− Difference = ∣μO2+−μO22−∣=∣3−0∣=3 B.M. ≈1.732 B.M.
-
Pair (4): O2−, O2+ Difference = ∣μO2−−μO2+∣=∣3−3∣=0 B.M.
Comparing the differences, the largest difference is 3 B.M., which occurs for the pair O2+ and O22− (Option 3).
