Question
Question: What will be the major product of the following reaction...
What will be the major product of the following reaction
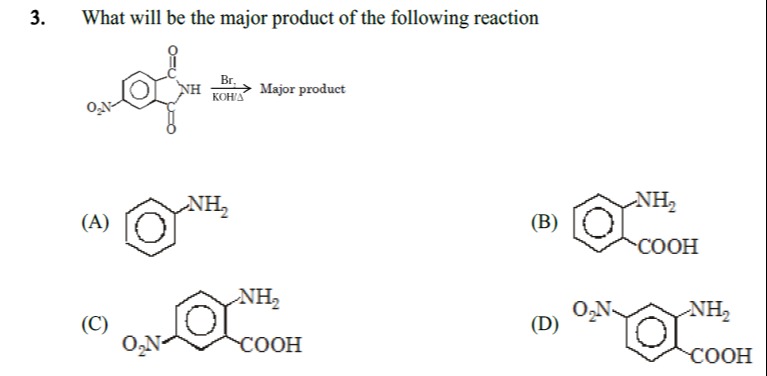
Aniline
Anthranilic acid (2-aminobenzoic acid)
2-amino-4-nitrobenzoic acid
2-amino-5-nitrobenzoic acid
2-amino-4-nitrobenzoic acid
Solution
The reaction shown is the Hofmann rearrangement of 4-nitrophthalimide. The Hofmann rearrangement converts primary amides into primary amines with one less carbon atom. In the case of cyclic imides like phthalimide, the reaction leads to the formation of an ortho-aminobenzoic acid.
The starting material is 4-nitrophthalimide. The structure of 4-nitrophthalimide can be represented as:
O
//
C
/ \
N-----C=O
/ \ /
O \ /
\ /
C1--C2
/ \
C6 C3
| |
C5----C4
|
NO2
In the Hofmann rearrangement, the aryl group migrates from the carbonyl carbon to the nitrogen atom. One of the carbonyl groups is lost as CO2, and the other becomes a carboxylic acid group. The nitrogen atom becomes an amino group. The product is an ortho-aminobenzoic acid.
The migration of the aryl group means that the nitrogen atom becomes directly bonded to the benzene ring. The nitro group, which is at position 4 of the benzene ring (relative to the attachment points of the imide), remains at its position.
Therefore, if we consider the carboxylic acid group to be at position 1 on the benzene ring, and the amino group to be at position 2 (ortho to the carboxylic acid), then the nitro group will be at position 4. This gives 2-amino-4-nitrobenzoic acid.
Let's examine the given options: (A) Aniline: This is incorrect as it lacks the nitro and carboxylic acid groups. (B) Anthranilic acid (2-aminobenzoic acid): This would be the product from unsubstituted phthalimide. (C) 2-amino-4-nitrobenzoic acid: This matches our prediction. The amino group is at position 2, the carboxylic acid group is at position 1, and the nitro group is at position 4. (D) 2-amino-5-nitrobenzoic acid: This would imply the nitro group is at position 5 relative to the amino and carboxylic acid groups. This would arise from a different starting isomer.
Thus, the major product of the reaction is 2-amino-4-nitrobenzoic acid.
