Question
Question: The product X is:...
The product X is:
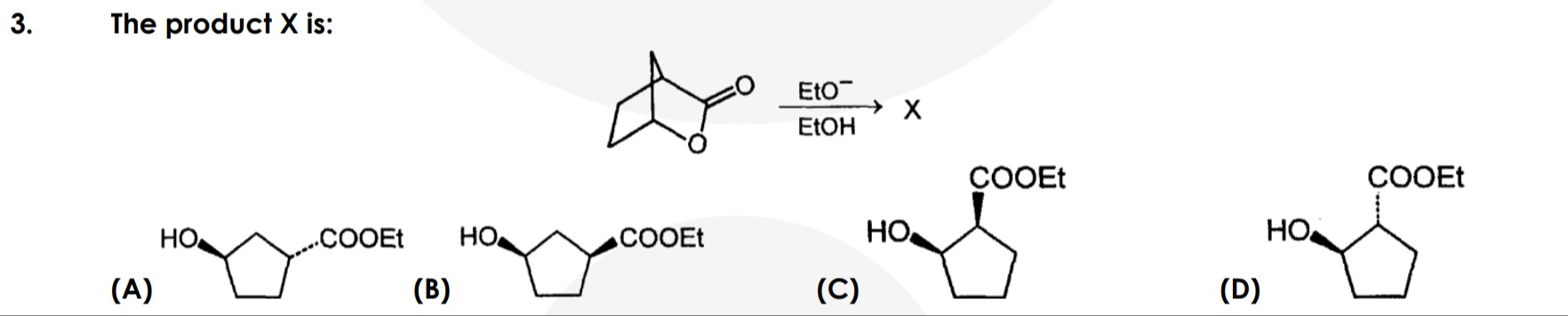
A
B
C
D
A
Solution
The starting material is a bicyclic lactone. Treatment with ethoxide (EtO−) in ethanol (EtOH) initiates a nucleophilic attack on the carbonyl carbon of the lactone. This opens the lactone ring, forming an alkoxide intermediate. Under these conditions, the bicyclic system undergoes a rearrangement. The ethoxide attacks the carbonyl carbon, leading to the opening of the lactone ring. A subsequent fragmentation of the bicyclic system occurs, leading to the formation of a cyclopentane derivative. Considering the stereochemistry depicted in the starting material and the typical outcomes of such rearrangements, product A is the most plausible outcome. The methyl group at the bridgehead influences the stereochemical outcome of the rearrangement. The ethoxide adds to the carbonyl, and the ring opens. A fragmentation of the bicyclic structure then leads to the cyclopentane ring. The stereochemistry of the resulting hydroxyl and ethoxycarbonyl groups is determined by the initial configuration of the bicyclic lactone and the mechanism of the rearrangement.
