Question
Question: Predict the major product (P) in the following reaction:...
Predict the major product (P) in the following reaction:
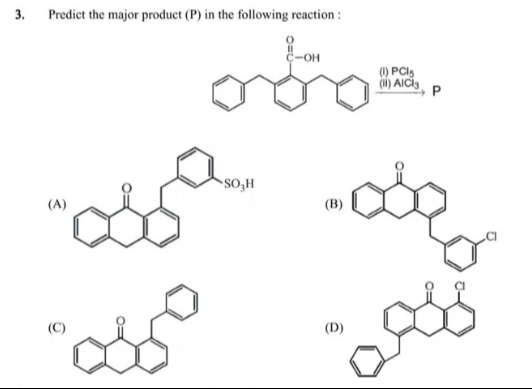
A
(A) a ketone with a sulfonic acid group attached to the phenyl ring
B
(B) a polyaromatic ketone bearing a chlorophenyl side chain
C
(C) a simple benzophenone derivative formed by intramolecular Friedel–Crafts acylation
D
(D) a chlorinated polyaromatic ketone with an external phenyl substituent
Answer
a simple benzophenone derivative formed by intramolecular Friedel–Crafts acylation
Explanation
Solution
Step 1: Conversion of –COOH to –COCl
Step 2: Intramolecular Friedel–Crafts acylation
- AlCl₃ activates the acyl chloride.
- The central ring undergoes electrophilic attack by the acylium ion.
- A new C–C bond forms, closing a ketone-containing ring.
Key points:
- PCl₅ efficiently converts carboxylic acids to acyl chlorides.
- Intramolecular acylation is favored when a suitably positioned aromatic ring can cyclize.
- No external chlorination or sulfonation occurs under these conditions.
