Question
Question: Observe the following Z vs P graph. The missing gas in the above graph can be :...
Observe the following Z vs P graph. The missing gas in the above graph can be :
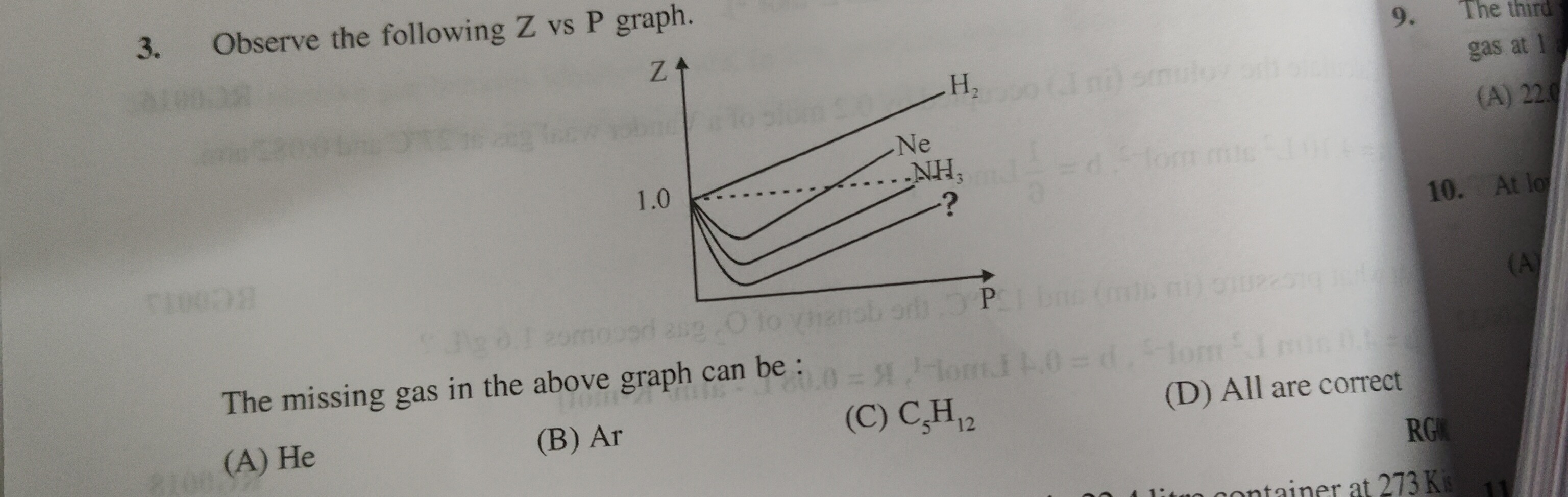
He
Ar
C₅H₁₂
All are correct
C₅H₁₂
Solution
The compressibility factor (Z) versus pressure (P) graph illustrates the deviation of real gases from ideal behavior. The deviation below Z=1 is primarily due to attractive intermolecular forces. A deeper dip in the Z vs P curve indicates stronger attractive forces. The graph shows the curves for H₂, Ne, NH₃, and a missing gas represented by '?'. The curves are ordered from top to bottom, implying an increase in the strength of attractive forces: H₂ < Ne < NH₃ < ?. We need to identify a gas from the options that has stronger attractive forces than NH₃.
Comparing the options:
- He: Has very weak intermolecular forces, weaker than H₂.
- Ar: Has stronger dispersion forces than Ne due to larger size, but generally weaker than the combined forces in NH₃ (which include dipole-dipole interactions and hydrogen bonding).
- C₅H₁₂ (Pentane): A larger hydrocarbon molecule with significant London dispersion forces, which are stronger than the attractive forces in NH₃.
Therefore, C₅H₁₂ would exhibit a deeper dip below Z=1 than NH₃, fitting the position of '?'.
