Question
Question: KI in acetone, undergoes S$_N$2 reaction with each of P, Q, R and S. The rates of the reaction vary ...
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S. The rates of the reaction vary as
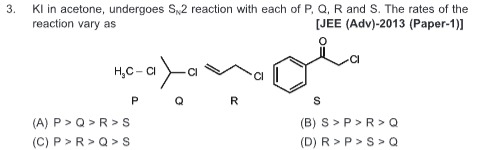
P > Q > R > S
S > P > R > Q
P > R > Q > S
R > P > S > Q
S > P > R > Q
Solution
The rate of an SN2 reaction is influenced by steric hindrance and electronic effects. The nucleophile (I−) and the solvent (acetone, a polar aprotic solvent) are constant.
- P: CH3-Cl (Methyl chloride): Minimal steric hindrance, highly reactive.
- Q: (CH3)2CH-Cl (Isopropyl chloride): Secondary halide, significant steric hindrance, less reactive than primary or methyl.
- R: CH2=CH-CH2-Cl (Allyl chloride): Primary allylic halide. Low steric hindrance, but the adjacent double bond provides resonance stabilization to the transition state, increasing reactivity compared to simple primary halides.
- S: Ph-CO-CH2-Cl (Phenacyl chloride): Alpha-halo ketone. The carbon bearing the chlorine is primary, but the adjacent carbonyl group is strongly electron-withdrawing, polarizing the C-Cl bond and making the carbon highly electrophilic. Resonance stabilization of the transition state further increases reactivity.
Comparing the substrates:
- Steric hindrance: Q (secondary) is more hindered than P (methyl), R (primary allylic), and S (primary alpha-halo ketone). Thus, Q is the least reactive.
- Electronic effects and resonance:
- S has a strongly electron-withdrawing carbonyl group and resonance stabilization, making it highly reactive, generally more reactive than methyl or allylic halides.
- R has resonance stabilization, making it more reactive than simple primary halides.
- P has minimal steric hindrance.
The general order of reactivity for SN2 reactions, considering these factors, is: Alpha-halo ketones (like S) > Allylic halides (like R) ≈ Methyl halides (like P) > Secondary halides (like Q). More precisely, the strong electron-withdrawing effect and resonance stabilization in S make it the most reactive. Methyl halides (P) are highly reactive due to minimal steric hindrance. Allylic halides (R) are more reactive than simple primary halides due to resonance. Secondary halides (Q) are the least reactive due to steric hindrance.
Therefore, the order of reactivity is S > P > R > Q.
