Question
Question: In which of the following reactions the system performs work on surrounding?...
In which of the following reactions the system performs work on surrounding?
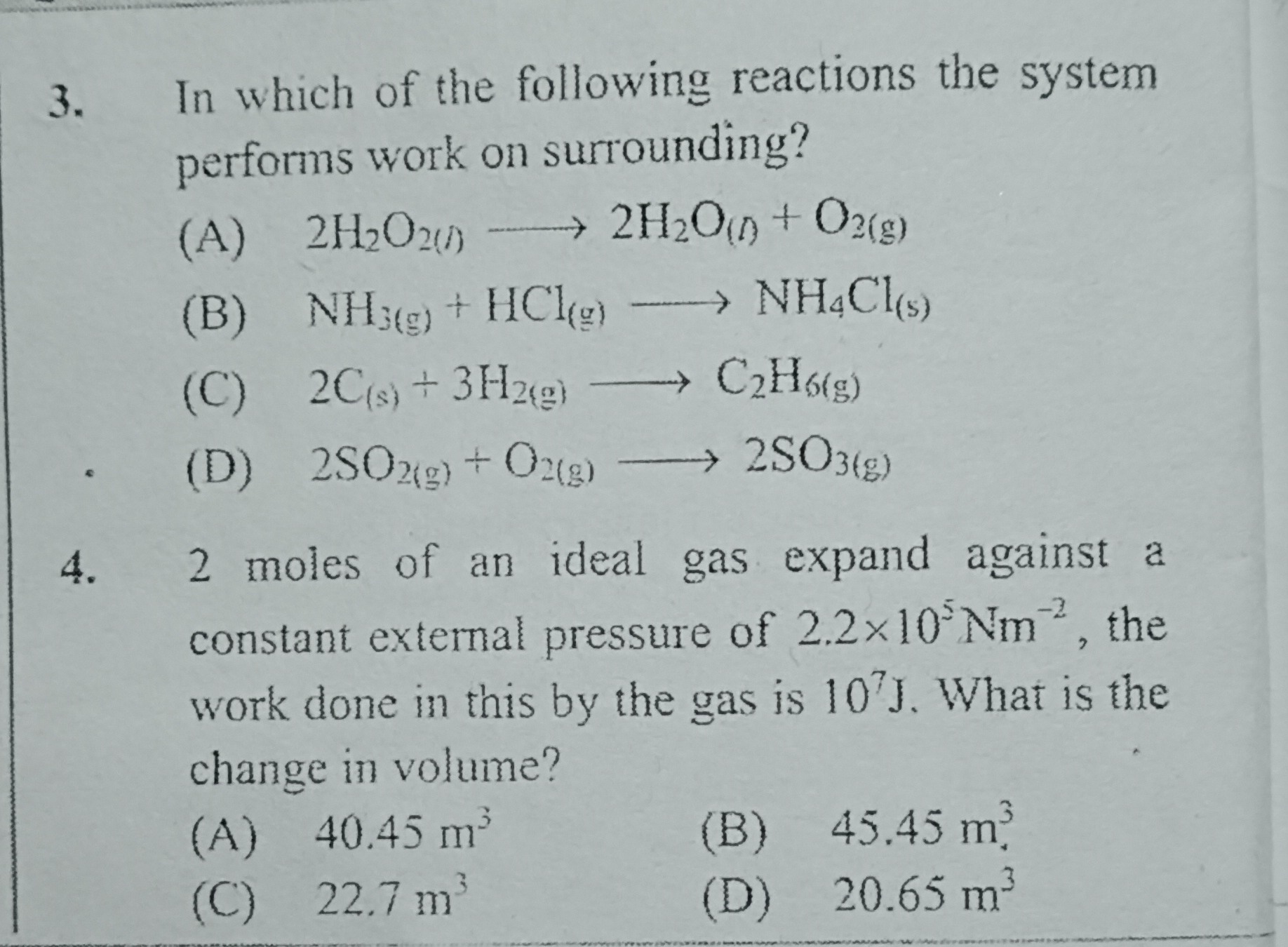
A
2H2O2(l) ⟶ 2H2O(l) + O2(g)
B
NH3(g) + HCl(g) ⟶ NH4Cl(s)
C
2C(s) + 3H2(g) ⟶ C2H6(g)
D
2SO2(g) + O2(g) ⟶ 2SO3(g)
Answer
2H2O2(l) ⟶ 2H2O(l) + O2(g)
Explanation
Solution
For the system to do expansion work on the surroundings, the reaction must result in an increase in the number of gas moles (i.e. expansion). In option (A), the production of gaseous O2 from liquid H2O2 causes an expansion in volume. In (B), the gaseous reactants form a solid; in (C) and (D), the reaction leads to a decrease in the number of gas moles.
