Question
Question: In the labelled N-atoms which is correct basic strength order : ...
In the labelled N-atoms which is correct basic strength order :
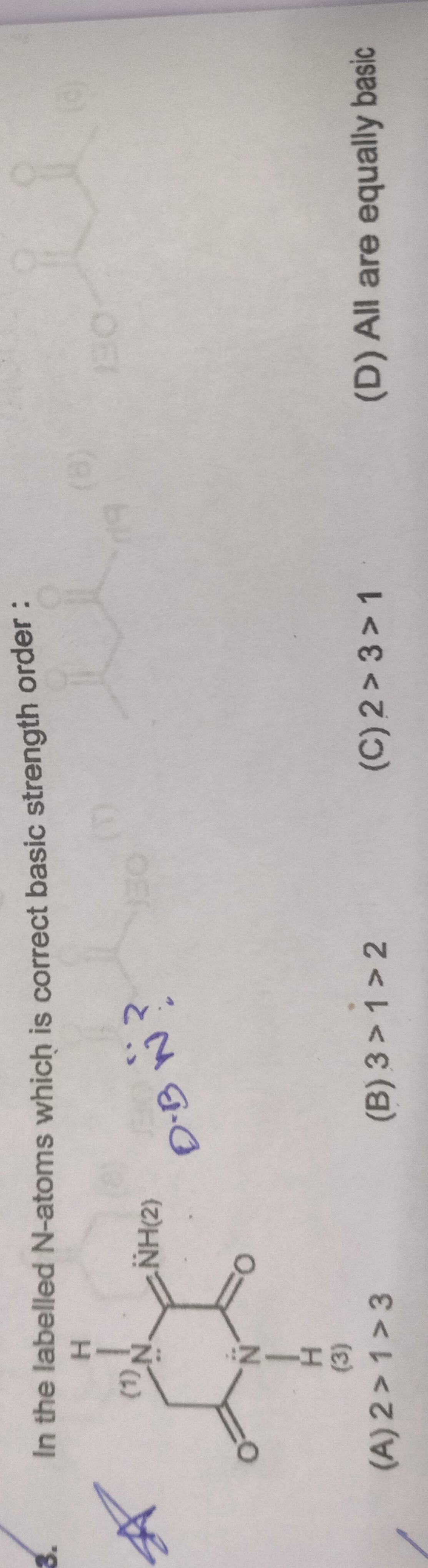
2 > 1 > 3
3 > 1 > 2
2 > 3 > 1
All are equally basic
A
Solution
The basic strength of a nitrogen atom depends on the availability of its lone pair of electrons for donation.
-
N(1) and N(3) are amide nitrogens. Their lone pairs are delocalized into one adjacent carbonyl group through resonance. This makes their lone pairs less available for protonation, thus reducing their basicity. Due to structural symmetry, N(1) and N(3) are electronically equivalent and should have similar basicity.
-
N(2) is an imine nitrogen (C=N-H). Its lone pair is located in an sp2 hybrid orbital and is perpendicular to the pi-system of the C=N bond. Therefore, this lone pair is localized on the nitrogen atom and is not involved in resonance with the adjacent carbonyl groups. This makes the lone pair on N(2) readily available for protonation.
Based on this, N(2) is significantly more basic than N(1) and N(3). So, the order of basic strength is N(2) > N(1) ≈ N(3). Among the given options, both (A) and (C) state that N(2) is the most basic. As N(1) and N(3) are equivalent, the order between them is arbitrary in the absence of other differentiating factors. Option (A) provides an order consistent with N(2) being the most basic.
