Question
Question: For an element, the covalent radius is xÅ, metallic radius is yÅ and the Vanderwaal's radius is zÅ. ...
For an element, the covalent radius is xÅ, metallic radius is yÅ and the Vanderwaal's radius is zÅ. Which of the following order is correct?
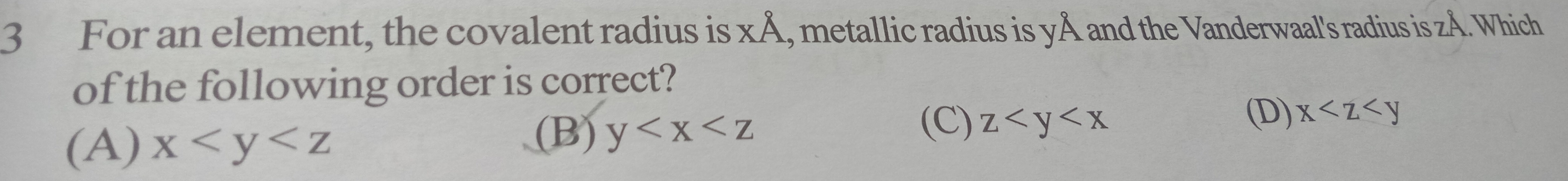
A
x<y<z
B
y<x<z
C
z<y<x
D
x<z<y
Answer
x<y<z
Explanation
Solution
The covalent radius is the smallest because it involves strong covalent bonding with significant orbital overlap. The metallic radius is larger as metallic bonding, while strong, typically results in slightly larger internuclear distances than covalent bonding for the same element. The Van der Waals radius is the largest because it represents the closest approach of non-bonded atoms, governed by weak Van der Waals forces. Thus, the order is covalent radius < metallic radius < Van der Waals radius.
