Question
Question: 3. ...
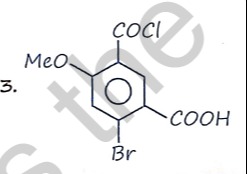
Answer
5-bromo-2-chloroformyl-3-methoxybenzoic acid
Explanation
Solution
The compound is a substituted benzoic acid. According to IUPAC nomenclature rules, the carboxylic acid group (-COOH) has the highest priority. The carbon atom attached to the -COOH group is designated as C1. Numbering proceeds around the benzene ring to give the lowest possible locants to the substituents.
The substituents and their positions are:
- Carboxylic acid (-COOH) at C1.
- Acid chloride (-COCl) at C2 (ortho to -COOH).
- Methoxy group (-OMe) at C3 (meta to -COOH).
- Bromine atom (-Br) at C5 (meta to -COOH).
The parent name is benzoic acid. The substituents are:
- Bromo at position 5.
- Chloroformyl at position 2 (the -COCl group is named as chloroformyl when it is a substituent).
- Methoxy at position 3.
These substituents are arranged alphabetically: bromo, chloroformyl, methoxy. Combining these with the parent name gives the IUPAC name: 5-bromo-2-chloroformyl-3-methoxybenzoic acid.
