Question
Question: Calculate the amount of He (in gm) present in the 10 litre container at 240 atm and 300K. Given valu...
Calculate the amount of He (in gm) present in the 10 litre container at 240 atm and 300K. Given value of "b" for He is 0.08 dm³ mol¯¹. (R = 0.08 atm lit mol' K¯¹)
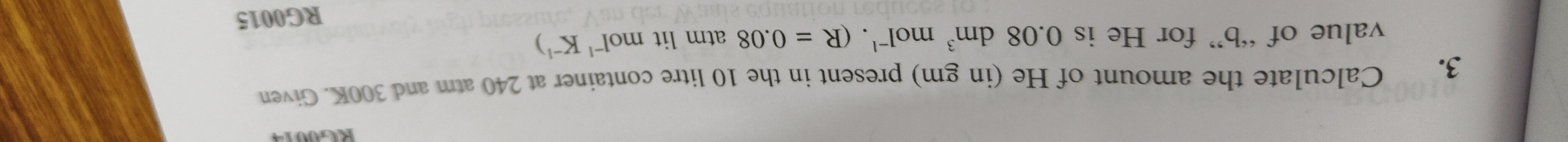
2000/9 gm
Solution
The van der Waals equation for a real gas is (P+V2an2)(V−nb)=nRT. Given that 'a' is not provided and pressure is high, we can approximate the equation to P(V−nb)=nRT. We are given: V=10 L P=240 atm T=300 K b=0.08 dm³ mol⁻¹ = 0.08 L mol⁻¹ R=0.08 atm L mol⁻¹ K⁻¹
Rearranging the simplified equation to solve for n: n=RT+PbPV
Calculate the terms: RT=(0.08 atm L mol−1 K−1)×(300 K)=24 atm L mol−1 Pb=(240 atm)×(0.08 L mol−1)=19.2 atm L mol−1 PV=(240 atm)×(10 L)=2400 atm L
Substitute these values: n=24 atm L mol−1+19.2 atm L mol−12400 atm L=43.22400 mol=9500 mol
The molar mass of Helium (He) is approximately 4 g/mol. Mass (m) = n×M=9500 mol×4 g/mol=92000 g.
