Question
Question: If the %-s-character in one Sb-H bond in SbH$_{3}$ is 1.0%. What is % p-character is the orbital occ...
If the %-s-character in one Sb-H bond in SbH3 is 1.0%. What is % p-character is the orbital occupied by its lone pair -
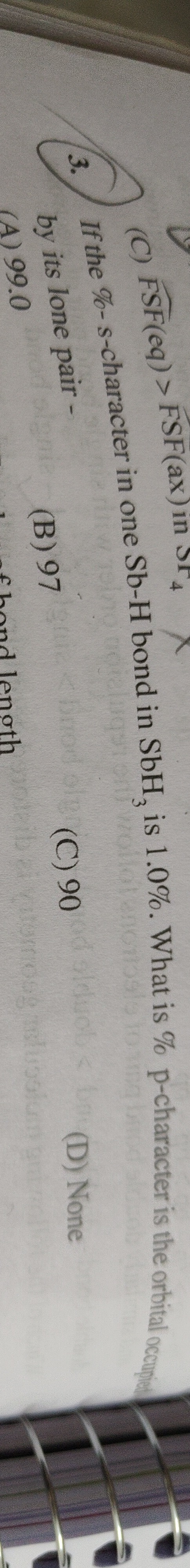
99.0
97
90
None
None
Solution
To determine the percentage of p-character in the orbital occupied by the lone pair in SbH₃, we use the principle of conservation of s and p character in hybrid orbitals.
-
Identify the central atom and its hybridization:
The central atom is Antimony (Sb), which belongs to Group 15. It forms three bonds with hydrogen atoms (Sb-H bonds) and has one lone pair of electrons. In a molecule like SbH₃, the central atom's valence orbitals (one s and three p) participate in hybridization to form the bonding orbitals and accommodate the lone pair. -
Conservation of s and p character:
For a central atom undergoing hybridization, the sum of the s-character of all hybrid orbitals must be equal to 1 (since only one s-orbital is involved in hybridization). Similarly, the sum of the p-character of all hybrid orbitals must be equal to 3 (since three p-orbitals are involved).Let:
s_bbe the s-character in each Sb-H bond orbital.p_bbe the p-character in each Sb-H bond orbital.s_lpbe the s-character in the lone pair orbital.p_lpbe the p-character in the lone pair orbital.
There are 3 Sb-H bond orbitals and 1 lone pair orbital.
- Total s-character:
3 * s_b + 1 * s_lp = 1 - Total p-character:
3 * p_b + 1 * p_lp = 3
-
Use the given information:
We are given that the %-s-character in one Sb-H bond is 1.0%.
So,s_b = 0.01. -
Calculate
s_lp:
Substitutes_binto the total s-character equation:
3 * (0.01) + s_lp = 1
0.03 + s_lp = 1
s_lp = 1 - 0.03 = 0.97 -
Calculate
p_lp:
For any hybrid orbital, the sum of its s-character and p-character is 1 (or 100%).
So, for the lone pair orbital:s_lp + p_lp = 1
0.97 + p_lp = 1
p_lp = 1 - 0.97 = 0.03 -
Convert to percentage:
The percentage p-character in the orbital occupied by the lone pair is0.03 * 100 = 3.0%. -
Compare with options:
The calculated value is 3.0%.
The given options are (A) 99.0, (B) 97, (C) 90, (D) None.
Since 3.0% is not among options A, B, or C, the correct choice is (D) None.
The final answer is None
Explanation of the solution:
The problem involves calculating the percentage p-character of the lone pair orbital in SbH₃ given the s-character of the Sb-H bond. We use the principle of conservation of s and p character. For a central atom, the sum of s-character in all hybrid orbitals equals 1, and the sum of p-character equals 3. Given 1% s-character in each of the three Sb-H bonds (s_b = 0.01), we calculate the s-character of the lone pair (s_lp) using 3 * s_b + s_lp = 1. This yields s_lp = 0.97. Since s_lp + p_lp = 1, the p-character of the lone pair (p_lp) is 1 - 0.97 = 0.03. As a percentage, this is 3.0%. Since this value is not among the given options, the answer is None.
