Question
Question: Analysis of a solution of Co₂(SO₄)₃ establishes that the concentration of SO₄²⁻ is 0.060 mol L⁻¹. Wh...
Analysis of a solution of Co₂(SO₄)₃ establishes that the concentration of SO₄²⁻ is 0.060 mol L⁻¹. What is the concentration of Co³⁺ in this solution?
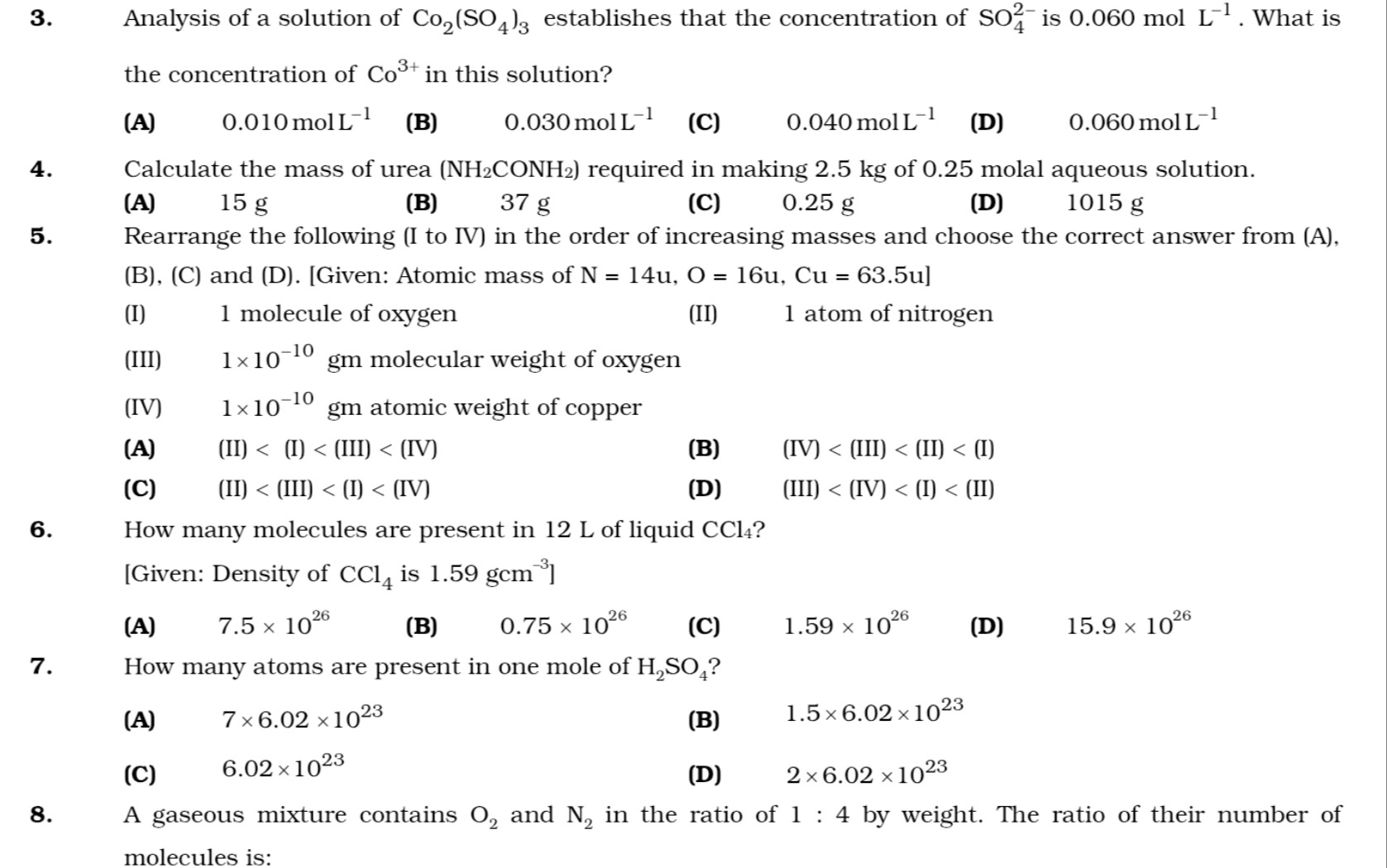
0.010 mol L⁻¹
0.030 mol L⁻¹
0.040 mol L⁻¹
0.060 mol L⁻¹
0.040 mol L⁻¹
Solution
The salt cobalt(III) sulfate, Co₂(SO₄)₃, dissociates in water according to the following ionic equation: Co2(SO4)3(s)→2Co3+(aq)+3SO42−(aq) This stoichiometry shows that for every 1 mole of Co₂(SO₄)₃ that dissolves, 2 moles of Co³⁺ ions and 3 moles of SO₄²⁻ ions are produced. Therefore, the molar ratio of Co³⁺ ions to SO₄²⁻ ions in the solution is 2:3.
Given the concentration of SO₄²⁻ is 0.060 mol L⁻¹. Let the concentration of Co³⁺ be CCo3+. Using the stoichiometric ratio: [SO42−][Co3+]=32 [Co3+]=32×[SO42−] [Co3+]=32×0.060 mol L−1 [Co3+]=0.040 mol L−1
