Question
Question: A thin tube of uniform cross section has trapped air columns of lengths 46 and 44.5 cm above and bel...
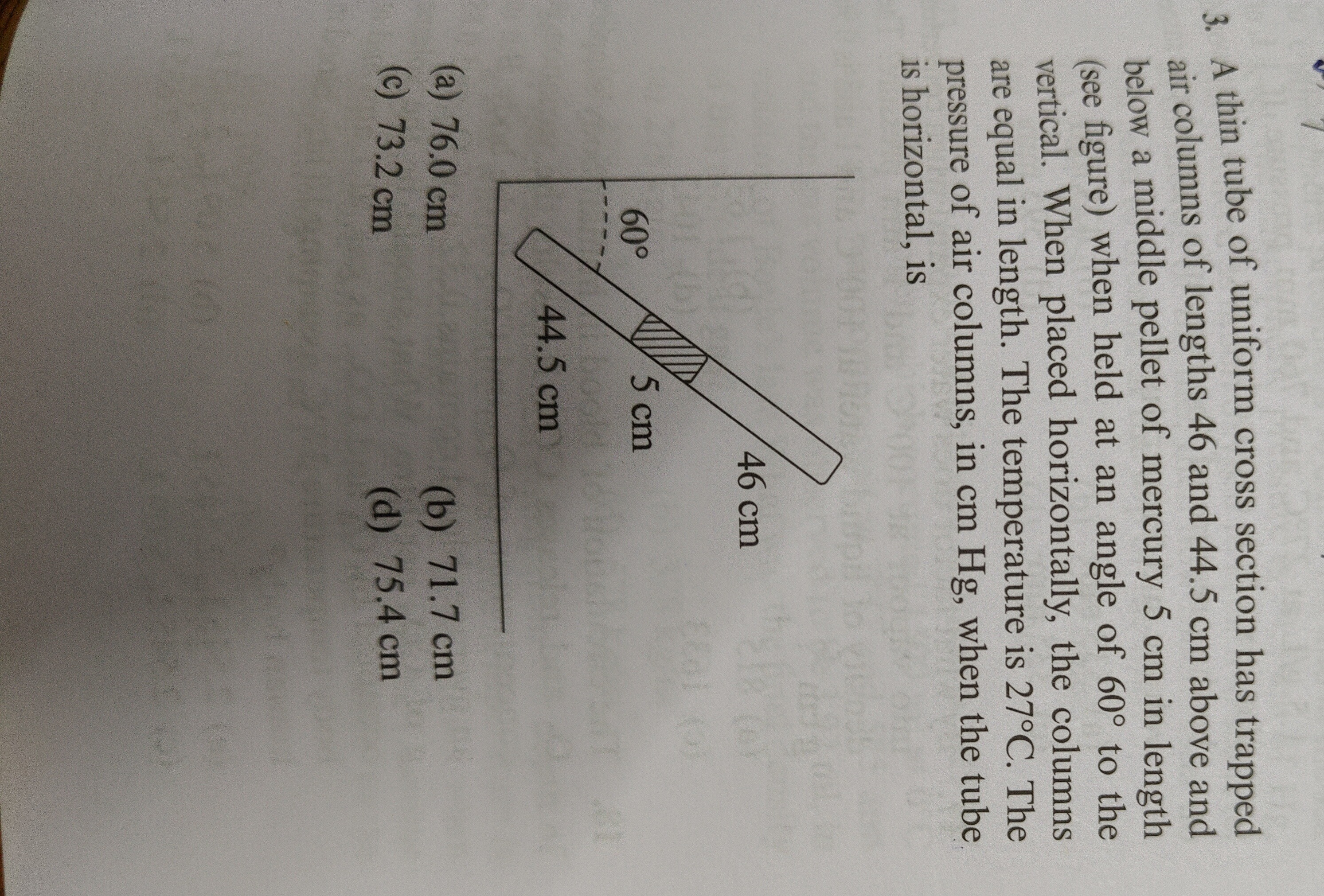
A thin tube of uniform cross section has trapped air columns of lengths 46 and 44.5 cm above and below a middle pellet of mercury 5 cm in length (see figure) when held at an angle of 60° to the vertical. When placed horizontally, the columns are equal in length. The temperature is 27°C. The pressure of air columns, in cm Hg, when the tube is horizontal, is
76.0 cm
71.7 cm
73.2 cm
75.4 cm
75.4 cm
Solution
Let P1 and P2 be the pressures of the air columns above and below the mercury pellet, respectively, when the tube is inclined. Let l1=46 cm and l2=44.5 cm be their lengths. The mercury pellet has length LHg=5 cm. The vertical height difference h across the mercury pellet is h=LHgcos(60∘)=5×21=2.5 cm. Thus, the pressure relation is P2=P1+h=P1+2.5 cm Hg.
When the tube is placed horizontally, the air columns are equal in length, say l′. The total length of trapped air is l1+l2=46+44.5=90.5 cm. When horizontal, the total length of air is 2l′. Thus, 2l′=90.5 cm, so l′=45.25 cm. Let P be the pressure of the air columns when the tube is horizontal. Since the columns are equal in length and the tube is horizontal, their pressures are equal, P1′=P2′=P.
By Boyle's Law (PV=constant at constant temperature): P1V1=P1′V1′⟹P1(46A)=P(45.25A)⟹46P1=45.25P P2V2=P2′V2′⟹P2(44.5A)=P(45.25A)⟹44.5P2=45.25P From these, we get 46P1=44.5P2.
We have a system of equations:
- P2=P1+2.5
- 46P1=44.5P2
Substitute (1) into (2): 46P1=44.5(P1+2.5) 46P1=44.5P1+111.25 1.5P1=111.25 P1=1.5111.25=6445 cm Hg.
Now, we find P using 46P1=45.25P: P=45.2546P1=45.2546×6445=45.2520470/6≈75.396 cm Hg.
