Question
Question: A carnot engine, whose source is at 400 K, takes 100 cal of heat and reflects 75 cal to the sink. Wh...
A carnot engine, whose source is at 400 K, takes 100 cal of heat and reflects 75 cal to the sink. What is temperature of the sink?
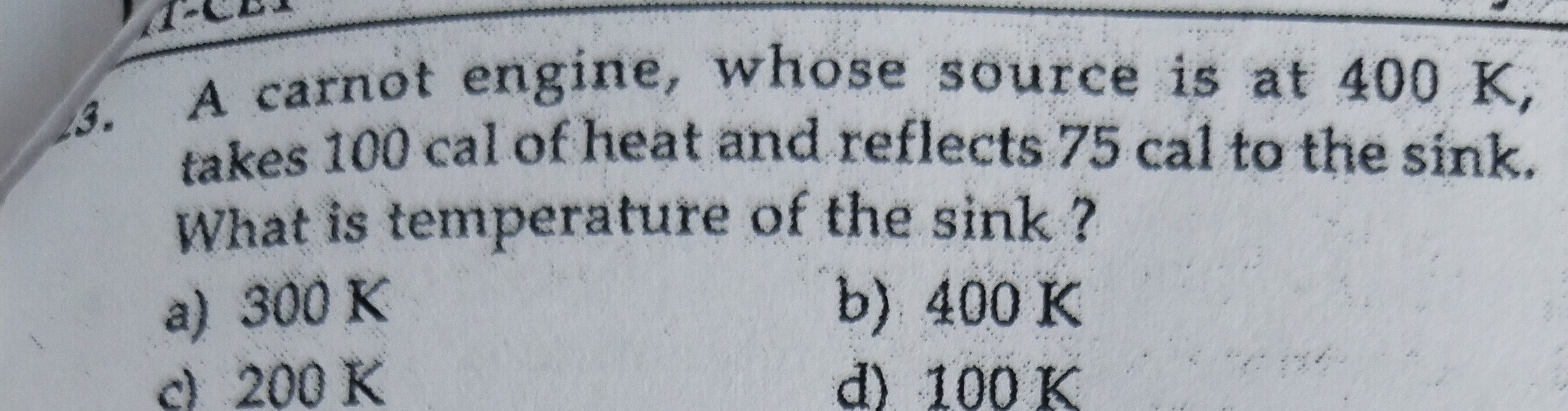
A
300 K
B
400 K
C
200 K
D
100 K
Answer
300 K
Explanation
Solution
The efficiency of a Carnot engine is given by:
η=1−TsourceTsink=1−QinQoutGiven:
- Tsource=400K
- Qin=100cal
- Qout=75cal
Calculate efficiency using heat quantities:
η=1−10075=1−0.75=0.25Now,
1−400Tsink=0.25⟹400Tsink=0.75 Tsink=400×0.75=300K