Question
Question: For the first order decomposition of SO₂Cl₂(g), SO₂Cl₂(g) → SO₂(g) + Cl₂(g) a graph of log (a₀ - x) ...
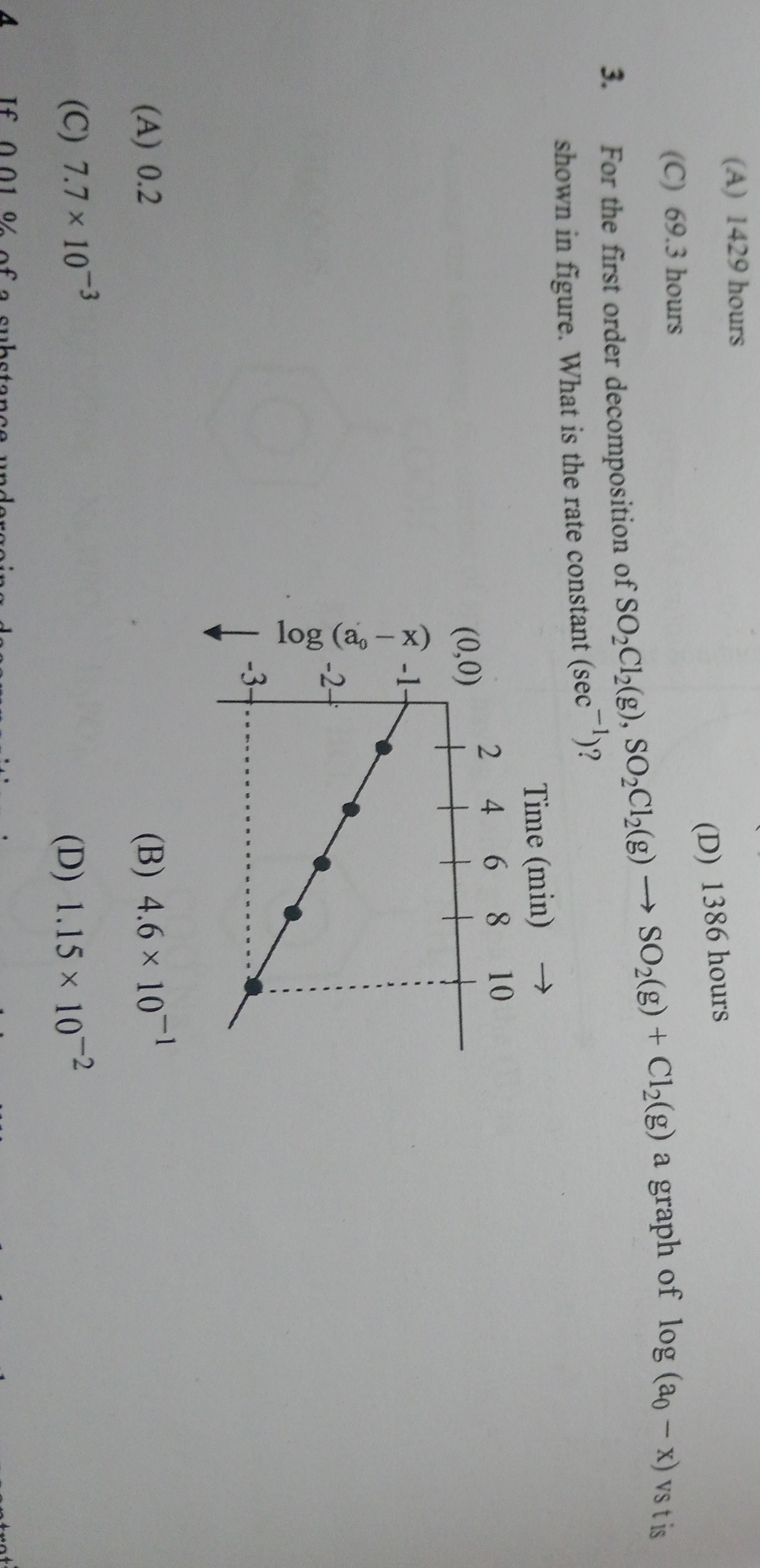
For the first order decomposition of SO₂Cl₂(g), SO₂Cl₂(g) → SO₂(g) + Cl₂(g) a graph of log (a₀ - x) vs t is shown in figure. What is the rate constant (sec⁻¹)?
0.2
4.6 × 10⁻¹
7.7 × 10⁻³
1.15 × 10⁻²
7.7 × 10⁻³
Solution
For a first-order reaction, the integrated rate law in terms of concentration at time t, [A]t, and initial concentration [A]0 is:
ln[A]t=−kt+ln[A]0
The given reaction is SO2Cl2(g)→SO2(g)+Cl2(g). Let the initial concentration of SO2Cl2 be a0 and the concentration at time t be (a0−x).
So, [A]t=(a0−x) and [A]0=a0.
The integrated rate law is:
ln(a0−x)=−kt+ln(a0)
The graph is given as log(a0−x) vs t. Assuming 'log' refers to log base 10, the equation is:
log10(a0−x)=−(k/2.303)∗t+log10(a0)
This is a linear equation of the form y=mx+c, where:
y=log10(a0−x) x=t m=−k/2.303 (slope) c=log10(a0) (y-intercept)
From the graph, the line passes through the points (0, 0) and (10, -1).
The y-intercept is 0, which means log10(a0)=0, so a0=100=1.
The slope of the line is calculated using the points (0, 0) and (10, -1):
Slope (m) = (y2−y1)/(x2−x1)=(−1−0)/(10−0)=−1/10=−0.1
From the equation, the slope is m=−k/2.303.
So, −0.1=−k/2.303 k=0.1∗2.303 min−1 k=0.2303 min−1
The question asks for the rate constant in sec−1. We need to convert the time unit from minutes to seconds.
1 minute = 60 seconds.
k (in sec−1) = k (in min−1) / 60 k=0.2303/60 sec−1
Calculating the value:
k≈0.003838 sec−1=3.838×10−3 sec−1
However, the provided options suggest the correct answer is 7.7×10−3 sec−1. This implies the slope of the graph is actually -0.2, not -0.1. If the slope is -0.2, then:
−0.2=−k/2.303 k=0.2∗2.303 min−1=0.4606 min−1 k=0.4606/60 sec−1≈0.007676 sec−1≈7.7×10−3 sec−1
This matches option (C). So, it is highly probable that the graph is drawn incorrectly, and the intended slope was -0.2.
