Question
Question: 2X (g)+Y(g) +3Z (g) → Products. The rate equation of above reaction is given by: Rate = K [X]¹ [Y]⁰ ...
2X (g)+Y(g) +3Z (g) → Products. The rate equation of above reaction is given by: Rate = K [X]¹ [Y]⁰ [Z]². Choose the correct statements
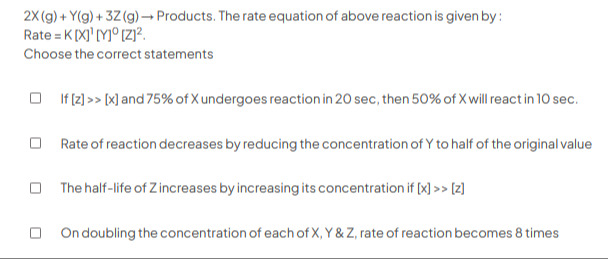
If [z] >> [x] and 75% of X undergoes reaction in 20 sec, then 50% of X will react in 10 sec.
Rate of reaction decreases by reducing the concentration of Y to half of the original value
The half-life of Z increases by increasing its concentration if [x] >> [z]
On doubling the concentration of each of X, Y & Z, rate of reaction becomes 8 times
- If [z] >> [x] and 75% of X undergoes reaction in 20 sec, then 50% of X will react in 10 sec.
- On doubling the concentration of each of X, Y & Z, rate of reaction becomes 8 times
Solution
The given rate equation is: Rate = K[X]1[Y]0[Z]2
This means the reaction is first order with respect to X, zero order with respect to Y, and second order with respect to Z.
-
Statement 1: "If [z] >> [x] and 75% of X undergoes reaction in 20 sec, then 50% of X will react in 10 sec." If [z] >> [x], the concentration of Z can be considered constant during the reaction with X. The rate law becomes Rate = K[X][Z]02=Keff[X], where Keff=K[Z]02 is a constant. This is a pseudo-first-order reaction with respect to X. For a first-order reaction, the integrated rate law is ln([X]0/[X])=Kefft. Time for 75% reaction: [X]=[X]0−0.75[X]0=0.25[X]0. ln([X]0/0.25[X]0)=Kefft75% ln(4)=Keff×20 2ln(2)=Keff×20⟹Keff=10ln(2) Time for 50% reaction (half-life, t1/2): [X]=[X]0−0.50[X]0=0.50[X]0. ln([X]0/0.50[X]0)=Kefft50% ln(2)=Kefft50% Substitute Keff: ln(2)=10ln(2)t50%⟹t50%=10 sec. The statement is correct.
-
Statement 2: "Rate of reaction decreases by reducing the concentration of Y to half of the original value" The rate law is Rate = K[X]1[Y]0[Z]2=K[X][Z]2. The rate of reaction is independent of the concentration of Y because the reaction order with respect to Y is 0. Reducing the concentration of Y will not change the rate, assuming [X] and [Z] are constant. The statement is incorrect.
-
Statement 3: "The half-life of Z increases by increasing its concentration if [x] >> [z]" If [x] >> [z], the concentration of X can be considered constant during the reaction with Z. The rate law becomes Rate = K[X]0[Z]2=Keff[Z]2, where Keff=K[X]0 is a constant. This is a pseudo-second-order reaction with respect to Z. For a second-order reaction (Rate = k′[A]2), the half-life is given by t1/2=k′[A]01. In this case, the half-life of Z is t1/2,Z=Keff[Z]01=K[X]0[Z]01. The half-life of Z is inversely proportional to its initial concentration [Z]0. Increasing [Z]0 will decrease the half-life of Z. The statement is incorrect.
-
Statement 4: "On doubling the concentration of each of X, Y & Z, rate of reaction becomes 8 times" Let the initial concentrations be [X]0, [Y]0, and [Z]0. Initial Rate = K[X]01[Y]00[Z]02. New concentrations are [X]′=2[X]0, [Y]′=2[Y]0, [Z]′=2[Z]0. New Rate = K[X′]1[Y′]0[Z′]2=K(2[X]0)1(2[Y]0)0(2[Z]0)2 New Rate = K(2[X]0)(1)(4[Z]02) New Rate = 8×K[X]0[Z]02 New Rate = 8 × Initial Rate. The rate of reaction becomes 8 times. The statement is correct.
The correct statements are 1 and 4.
Explanation: The rate law is Rate = K[X]1[Y]0[Z]2. Statement 1: Under the condition [Z] >> [X], the reaction is pseudo-first order w.r.t. X. For a first-order reaction, t75%=2×t50%. Given t75%=20s, t50%=10s. Correct. Statement 2: The rate is independent of [Y] as the order w.r.t. Y is 0. Incorrect. Statement 3: Under the condition [X] >> [Z], the reaction is pseudo-second order w.r.t. Z. For a second-order reaction, t1/2∝1/[reactant]0. Increasing [Z]₀ decreases t1/2. Incorrect. Statement 4: Rate ∝[X]1[Z]2. Doubling [X], [Y], [Z] changes rate by (2)1×(2)0×(2)2=2×1×4=8 times. Correct.
