Question
Question: $2MnO_4^- + b \ C_2O_4^{2-} + c \ H^+ \rightarrow x \ Mn^{2+} + y \ CO_2 + z \ H_2O$ If the above e...
2MnO4−+b C2O42−+c H+→x Mn2++y CO2+z H2O
If the above equation is balanced with integer coefficients, the value of c is ........ (Round off to the Nearest Integer)
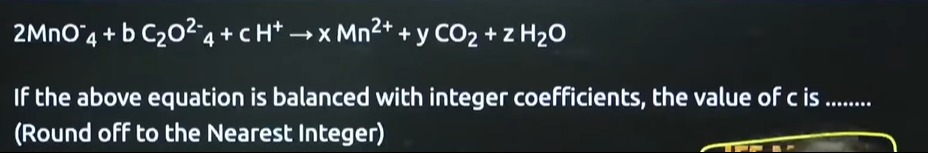
16
Solution
1. Identify Half-Reactions:
- Reduction: MnO4− (Mn oxidation state +7) is reduced to Mn2+ (Mn oxidation state +2).
- Oxidation: C2O42− (C oxidation state +3) is oxidized to CO2 (C oxidation state +4).
2. Balance Half-Reactions:
-
Reduction: MnO4−→Mn2+ Balance O by adding H2O: MnO4−→Mn2++4H2O Balance H by adding H+: MnO4−+8H+→Mn2++4H2O Balance charge by adding electrons (e−): MnO4−+8H++5e−→Mn2++4H2O (Charge: Left = -1+8 = +7; Right = +2)
-
Oxidation: C2O42−→CO2 Balance C: C2O42−→2CO2 Balance charge by adding electrons (e−): C2O42−→2CO2+2e− (Charge: Left = -2; Right = 0)
3. Equalize Electrons and Combine: To balance the electrons, multiply the reduction half-reaction by 2 and the oxidation half-reaction by 5:
- 2×(MnO4−+8H++5e−→Mn2++4H2O)⟹2MnO4−+16H++10e−→2Mn2++8H2O
- 5×(C2O42−→2CO2+2e−)⟹5C2O42−→10CO2+10e−
Add the two balanced half-reactions: (2MnO4−+16H++10e−)+(5C2O42−)→(2Mn2++8H2O)+(10CO2+10e−) Cancel electrons: 2MnO4−+5C2O42−+16H+→2Mn2++10CO2+8H2O
4. Identify Coefficient 'c': The balanced equation is 2MnO4−+5C2O42−+16H+→2Mn2++10CO2+8H2O. Comparing this with the given equation 2MnO4−+b C2O42−+c H+→x Mn2++y CO2+z H2O, we find that the coefficient of H+ is c=16.
5. Rounding: The question asks to round off the value of c to the nearest integer. Since c=16 is already an integer, the rounded value is 16.
