Question
Question: 2KMnO4 + 16HCl X + KCl + 8H2O + 5Cl2 The oxidation state of Mn in 'X' is:...
2KMnO4 + 16HCl X + KCl + 8H2O + 5Cl2 The oxidation state of Mn in 'X' is:
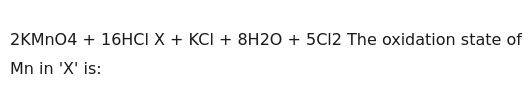
+2
Solution
The given chemical reaction is:
2KMnO4+16HCl→X+KCl+8H2O+5Cl2
To find the oxidation state of Mn in 'X', we first need to identify the chemical formula of 'X' by balancing the atoms on both sides of the equation.
-
Initial Atom Count (Reactants):
- K: 2
- Mn: 2
- O: 2×4=8
- H: 16
- Cl: 16
-
Initial Atom Count (Known Products):
- K: 1 (from KCl)
- H: 8×2=16 (from 8H2O)
- O: 8×1=8 (from 8H2O)
- Cl: 1 (from KCl) + 5×2=10 (from 5Cl2) = 11
-
Balance Potassium (K):
There are 2 K atoms on the reactant side (2KMnO4) but only 1 K atom in the known products (KCl). To balance K, we need 2 molecules of KCl. The equation becomes: 2KMnO4+16HCl→X+2KCl+8H2O+5Cl2
-
Revised Atom Count (Known Products with 2KCl):
- K: 2 (from 2KCl)
- H: 16 (from 8H2O)
- O: 8 (from 8H2O)
- Cl: 2 (from 2KCl) + 10 (from 5Cl2) = 12
-
Determine the atoms in 'X':
Compare the revised atom counts of known products with the reactant counts:
- Mn: Reactants have 2 Mn, known products have 0 Mn. So, X must contain 2 Mn atoms.
- Cl: Reactants have 16 Cl, known products have 12 Cl. So, X must contain 16−12=4 Cl atoms.
- All other atoms (K, H, O) are already balanced.
Therefore, the formula of 'X' is Mn2Cl4. This can be simplified to 2MnCl2, which means 'X' is MnCl2.
-
Calculate the oxidation state of Mn in 'X' (MnCl2):
In MnCl2, chlorine (Cl) typically has an oxidation state of -1. Let the oxidation state of Mn be 'y'. y+2×(−1)=0 y−2=0 y=+2
Thus, the oxidation state of Mn in 'X' (MnCl2) is +2.
The balanced chemical equation is: 2KMnO4+16HCl→2MnCl2+2KCl+8H2O+5Cl2
The oxidation state of Mn changes from +7 in KMnO4 to +2 in MnCl2.
