Question
Question: 296. <figure/>...
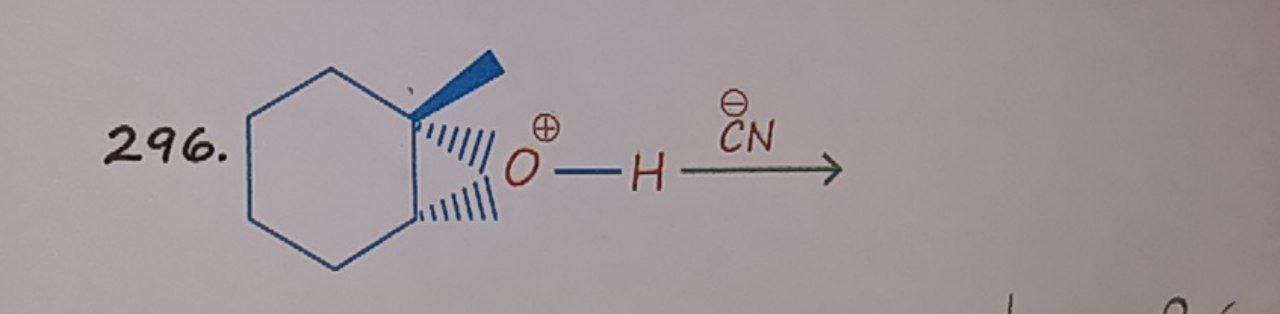
The product is a cyclohexane derivative with a hydroxyl group and a cyano group on adjacent carbons, and a methyl group on the carbon with the hydroxyl group. The relative stereochemistry is trans between the methyl and hydroxyl groups, and trans between the hydroxyl and cyano groups.
Solution
The reaction involves the acid-catalyzed opening of an epoxide by a cyanide nucleophile. The epoxide is on a cyclohexane ring and is substituted with a methyl group.
-
Protonation: The epoxide oxygen is protonated by the acidic medium (H₂O⁺). This activates the epoxide ring towards nucleophilic attack by increasing the electrophilicity of the epoxide carbons.
-
Nucleophilic Attack: The cyanide ion (CN⁻) is a strong nucleophile. It attacks one of the epoxide carbons. In acid-catalyzed epoxide opening, the nucleophile typically attacks the less substituted carbon to minimize steric hindrance, especially if SN2-like character dominates. In this case, one epoxide carbon is substituted with a methyl group, while the other is not. Therefore, the cyanide ion attacks the carbon without the methyl group.
-
Stereochemistry: The nucleophilic attack proceeds via an SN2 mechanism, which involves backside attack and leads to inversion of configuration at the attacked carbon. The epoxide ring opens, and the C-O bond breaks.
-
Regiochemistry and Stereochemistry Analysis: Let's analyze the given stereochemistry. The methyl group is shown with a wedge bond, indicating it is pointing out of the plane of the cyclohexane ring. The hatching lines on the epoxide bond indicate that the epoxide ring is oriented on the opposite side of the ring from the methyl group. Thus, if the methyl group is pointing "up", the epoxide oxygen is pointing "down".
The cyanide ion attacks the less substituted carbon (the one without the methyl group) from the backside. This means the attack occurs from the "top" side of the ring.
- The C-O bond on the attacked carbon breaks.
- The cyanide group attaches to this carbon, and due to backside attack, it will have the opposite orientation to the C-O bond that broke. If the epoxide oxygen was pointing "down" at this carbon, the cyanide will end up pointing "up".
- The oxygen atom remains attached to the other epoxide carbon (the one with the methyl group). It gets a proton and forms a hydroxyl group. Since the epoxide oxygen was pointing "down" at this carbon, the resulting hydroxyl group will also be pointing "down".
Therefore, at the carbon bearing the methyl group, we will have a methyl group pointing "up" and a hydroxyl group pointing "down". These are trans to each other. At the adjacent carbon, we will have a cyano group pointing "up".
The product will have a hydroxyl group and a cyano group on adjacent carbons, with a methyl group on the carbon bearing the hydroxyl group. The relative stereochemistry is such that the methyl and hydroxyl groups are trans, and the hydroxyl and cyano groups are trans.
