Question
Question: 29. Which of the following will not show optical isomerism?...
- Which of the following will not show optical isomerism?
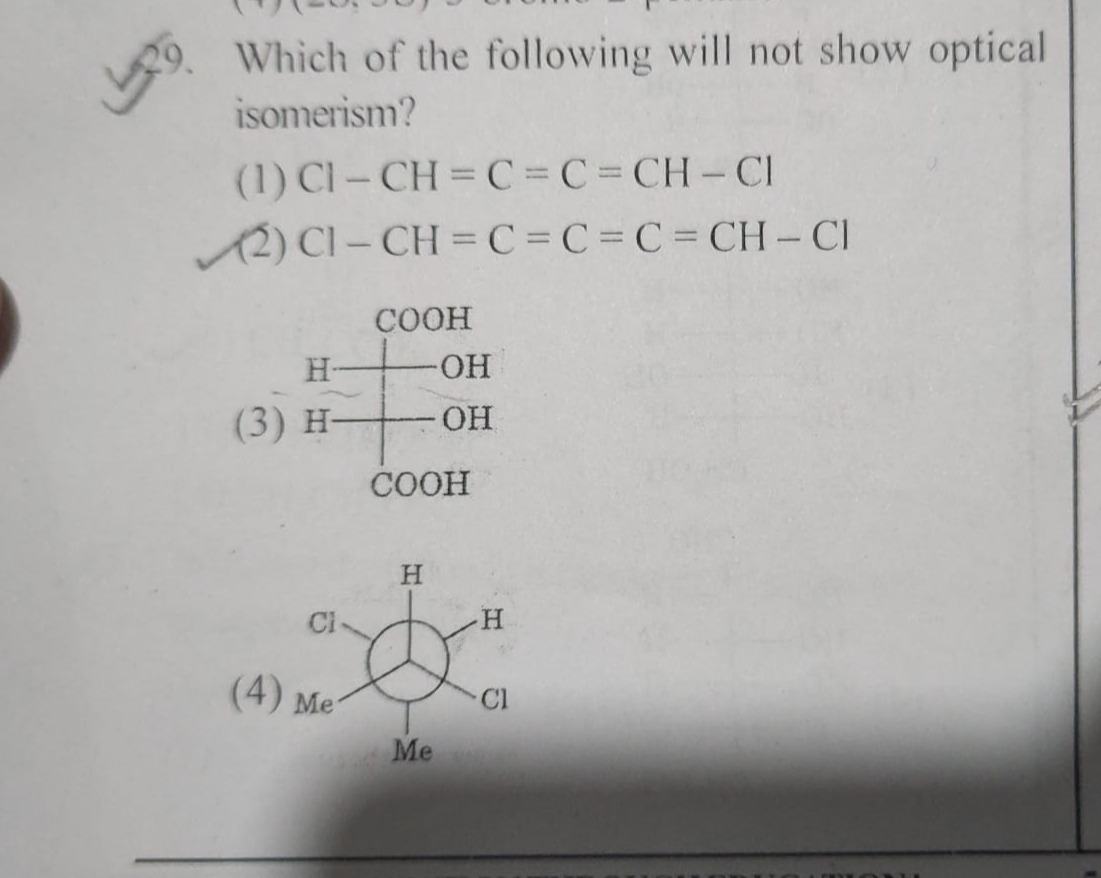
A
Cl - CH = C = C = CH - Cl
B
Cl - CH = C = C = C = CH - Cl
C
COOH H-OH H-OH COOH
D
H Ci H Me Cl Me
Answer
Option (1)
Explanation
Solution
For cumulenes, the chirality depends on the number of cumulated double bonds. In an allene (three carbons, two double bonds) with different substituents the molecule is chiral. However, in a cumulene with an even number of carbon atoms (like Cl–CH=C=C=CH–Cl, which has four carbons and three double bonds), the terminal substituents lie in the same plane due to orbital symmetry. This makes the molecule achiral, so it does not exhibit optical isomerism. The other compounds have stereogenic centers or arrangements that can give rise to enantiomers.
