Question
Question: Most acidic compound among the following is;...
Most acidic compound among the following is;
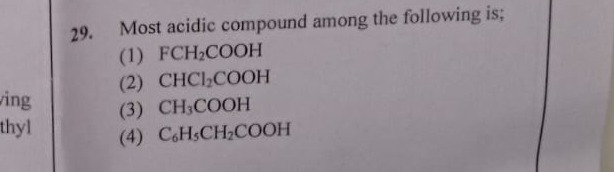
A
FCH2COOH
B
CHCl2COOH
C
CH3COOH
D
C6H5CH2COOH
Answer
CHCl2COOH
Explanation
Solution
Acidity of carboxylic acids is determined by the stability of their conjugate base (carboxylate ion). Electron-withdrawing groups (EWGs) stabilize the carboxylate ion by delocalizing the negative charge, thus increasing acidity. Electron-donating groups (EDGs) destabilize the carboxylate ion, decreasing acidity.
The strength of the electron-withdrawing effect depends on:
- Electronegativity of the atom: Higher electronegativity leads to a stronger -I effect (e.g., F > Cl > Br > I).
- Number of electron-withdrawing groups: More EWGs lead to a stronger cumulative effect.
- Distance from the -COOH group: Closer EWGs have a stronger effect.
Comparing the options: CHCl2COOH has two chlorine atoms, resulting in a strong cumulative electron-withdrawing effect, making it the most acidic.
