Question
Question: Consider the following base of RNA. Correct order of acidic strength on different sites will be:...
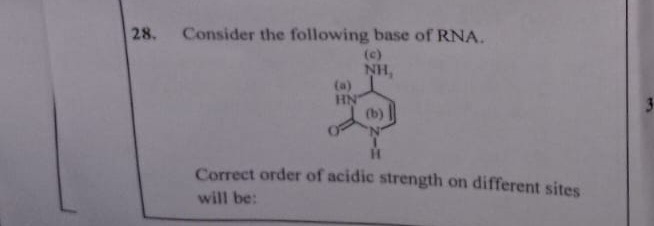
Consider the following base of RNA.
Correct order of acidic strength on different sites will be:
a > c > b
Solution
To determine the order of acidic strength of the protons at sites (a), (b), and (c) in the given RNA base (Cytosine), we need to evaluate the stability of the conjugate base formed after the removal of a proton from each site. A more stable conjugate base corresponds to a stronger acid.
Let's analyze each site:
1. Site (a): HN- (N1-H proton)
-
This proton is attached to a nitrogen atom (N1) that is part of a cyclic amide-like structure. The nitrogen is directly adjacent to a carbonyl group (C2=O).
-
Upon deprotonation, a negative charge forms on the nitrogen atom (N1).
-
This negative charge is highly stabilized by resonance with the adjacent carbonyl group. The electron density can be delocalized onto the electronegative oxygen atom of the carbonyl group.
\text{O=C--N--H} \quad \xrightarrow{-\text{H}^+} \quad \text{O=C--N}^- \quad \longleftrightarrow \quad \text{O}^-\text{--C=N} -
This type of proton (amide N-H) is relatively acidic. For cytosine, the pKa of this proton (N1-H) is reported to be around 12.16.
2. Site (c): NH₂ (Exocyclic amino group proton)
- This proton is attached to a nitrogen atom in the exocyclic amino group.
- Upon deprotonation, a negative charge forms on this nitrogen atom.
- The negative charge can be delocalized into the aromatic ring system. However, the amino group itself is generally electron-donating and basic.
- Compared to the resonance stabilization with a carbonyl group (as in site a), the delocalization into the aromatic ring for an exocyclic amine is less effective in stabilizing the negative charge on nitrogen.
- Amino groups are typically basic, meaning their conjugate acids (protonated amines) are acidic, but the N-H protons of the neutral amine are very weakly acidic. For aromatic amines like aniline, the pKa for deprotonation of the N-H bond is very high (typically > 30).
3. Site (b): CH=CH- (C5-H proton)
- This proton is attached to a carbon atom (C5) that is sp² hybridized and part of a double bond within the ring. This is a vinylic proton.
- Upon deprotonation, a negative charge forms on this carbon atom, creating a carbanion.
- Carbanions are generally less stable than anions on more electronegative atoms like nitrogen or oxygen.
- While some delocalization might occur within the ring, it is generally not very effective for stabilizing a carbanion compared to resonance with a carbonyl group or delocalization on a more electronegative atom.
- Vinylic protons are very weakly acidic, with typical pKa values in the range of 40-45.
Order of Acidic Strength:
Comparing the pKa values and stability of the conjugate bases:
- (a) HN-: Highly stabilized by resonance with carbonyl. (pKa ≈ 12)
- (c) NH₂: Less stabilized. Nitrogen is less electronegative than oxygen, and delocalization is less effective than with a carbonyl. (pKa > 30)
- (b) CH=CH-: Least stable, as it forms a carbanion on an sp² carbon. (pKa ≈ 40-45)
Therefore, the order of acidic strength from strongest to weakest is: (a) > (c) > (b)
The acidic strength is determined by the stability of the conjugate base.
- Deprotonation at site (a) forms an anion on nitrogen, which is highly stabilized by resonance with the adjacent carbonyl group, making it the most acidic.
- Deprotonation at site (c) forms an anion on the amino nitrogen. While some resonance into the ring is possible, it is less effective than stabilization by a carbonyl, and amino groups are generally weak acids.
- Deprotonation at site (b) forms a carbanion on an sp² hybridized carbon. Carbanions are generally unstable, making this the least acidic site.
Thus, the order of acidic strength is (a) > (c) > (b).
