Question
Question: At 323 K, the vapour pressure in millimeters of mercury of a methanol-ethanol solution is represente...
At 323 K, the vapour pressure in millimeters of mercury of a methanol-ethanol solution is represented by the equation, p = 120 XA + 140, where XA is the mole fraction of methanol. Then the value of limxA→1XApA is (where PA is vapour pressure of A)
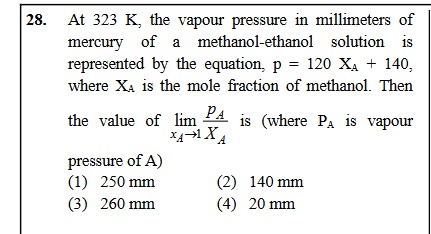
250 mm
140 mm
260 mm
20 mm
260 mm
Solution
The given equation for the total vapour pressure (p) of a methanol-ethanol solution at 323 K is: p=120XA+140 where XA is the mole fraction of methanol (component A).
For an ideal binary solution, the total vapour pressure is described by Raoult's Law: p=pA∗XA+pB∗XB where pA∗ and pB∗ are the vapour pressures of pure components A and B, respectively. Since XA+XB=1, we have XB=1−XA. Substituting this into Raoult's Law: p=pA∗XA+pB∗(1−XA) p=(pA∗−pB∗)XA+pB∗
Comparing this ideal solution equation with the given equation (p=120XA+140): By comparing the constant terms, we identify the vapour pressure of pure component B (ethanol) as: pB∗=140 mm
By comparing the coefficients of XA, we get: pA∗−pB∗=120 mm Substituting the value of pB∗: pA∗−140=120 pA∗=120+140=260 mm This is the vapour pressure of pure component A (methanol).
The question asks for the value of limXA→1XApA, where pA is the vapour pressure of A (methanol). For an ideal solution, the partial vapour pressure of component A is given by: pA=pA∗XA
Substituting this expression for pA into the limit: limXA→1XApA∗XA
Since XA→1, XA is not zero, so we can cancel XA: limXA→1pA∗
The vapour pressure of pure methanol, pA∗, is a constant value (260 mm). The limit of a constant is the constant itself. Therefore, limXA→1pA∗=pA∗=260 mm.
