Question
Chemistry Question on Thermodynamics
28.0L of CO2 is produced on complete combustion of 168L gaseous mixture of ethene and methane at 25∘C and 1atm Heat evolved during the combustion process is ______kJ Given : ΔHc(CH4)=−900kJmol−1 ΔHc(C2H4)=−1400kJmol−1
Answer
The correct answer is 925.
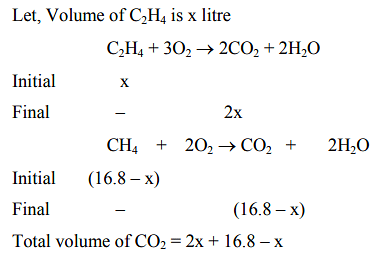
⇒28=16.8+x
x=11.2L
nCH4=RTPV=0.082×2981×5.6=0.229 mole
nC2H2=0.082×29811.2=0.458 mole
∴ Heat evolved =0.229×900+0.458×1400
=206.1+641.2
=847.3kJ
