Question
Question: What is number of moles of electrons gained by one mole of oxidizing agent in the following reaction...
What is number of moles of electrons gained by one mole of oxidizing agent in the following reaction?
Mn(aq)2++2ClO3(aq)⟶MnO2(s)+2ClO2(aq)
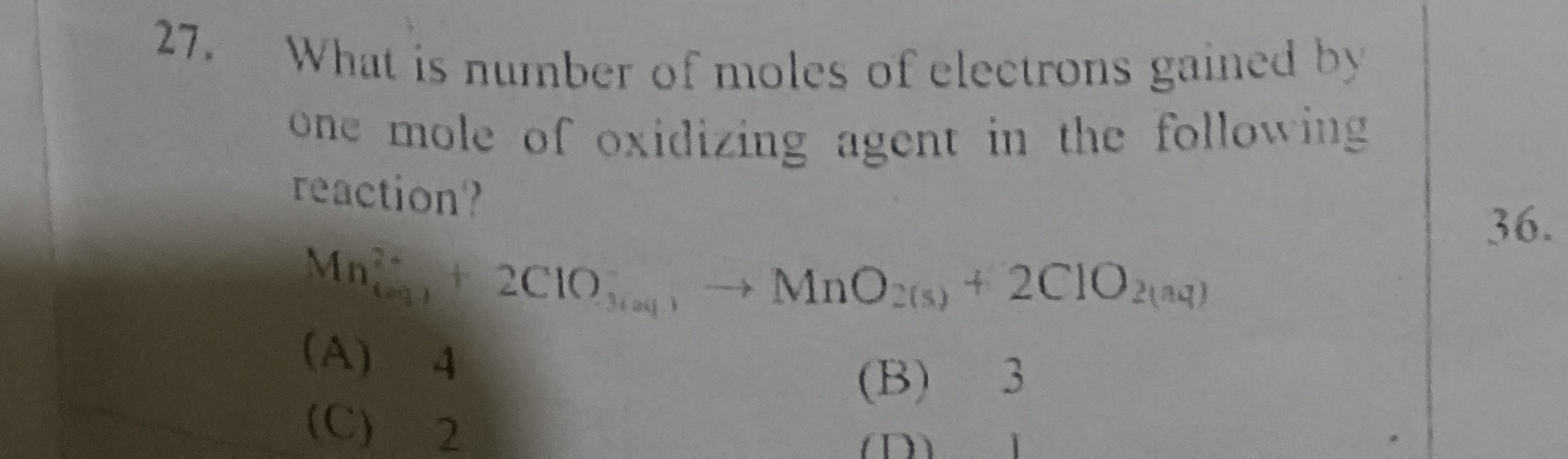
A
4
B
3
C
2
Answer
2
Explanation
Solution
-
Identify the oxidizing agent: In a redox reaction the oxidizing agent is reduced. Here, the chlorine‐containing species is converted from \ceClO3 to \ceClO2.
-
Assign oxidation numbers (using the neutral molecule interpretation): For a neutral molecule \ceClO3:
For \ceClO2:
x+2(−2)=0⟹x=+4.- Determine the change in oxidation state: Chlorine goes from +6 in \ceClO3 to +4 in \ceClO2. Thus, each chlorine atom gains
- Answer the question: Hence, one mole of the oxidizing agent (i.e. one mole of \ceClO3) gains 2 moles of electrons.
