Question
Question: The major product in the following reaction is: Cl 1. CH$_3$MgBr, dry either, 0°C 2. aq. acid O CH$...
The major product in the following reaction is:
Cl
- CH3MgBr, dry either, 0°C
- aq. acid O CH3
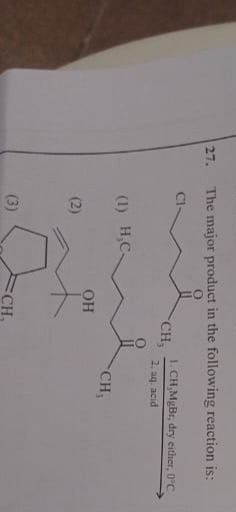
A
H3C
O CH3
B
OH
C
CH3
Answer
2,2-dimethyltetrahydrofuran
Explanation
Solution
The reaction proceeds in two main steps. First, the Grignard reagent (CH3MgBr) performs a nucleophilic addition to the ketone carbonyl group, forming an alkoxide intermediate. Second, this alkoxide undergoes an intramolecular SN2 reaction by attacking the primary alkyl chloride, leading to the formation of a stable 5-membered cyclic ether (2,2-dimethyltetrahydrofuran). The final aqueous acid workup protonates any remaining alkoxide or hydrolyzes the Mg-complex but does not alter the cyclic ether.
