Question
Question: Selected bond angles for six hydrocarbons are shown below. Arrange these hydrocarbons according to t...
Selected bond angles for six hydrocarbons are shown below. Arrange these hydrocarbons according to their pKa values, from the lowest to the highest.
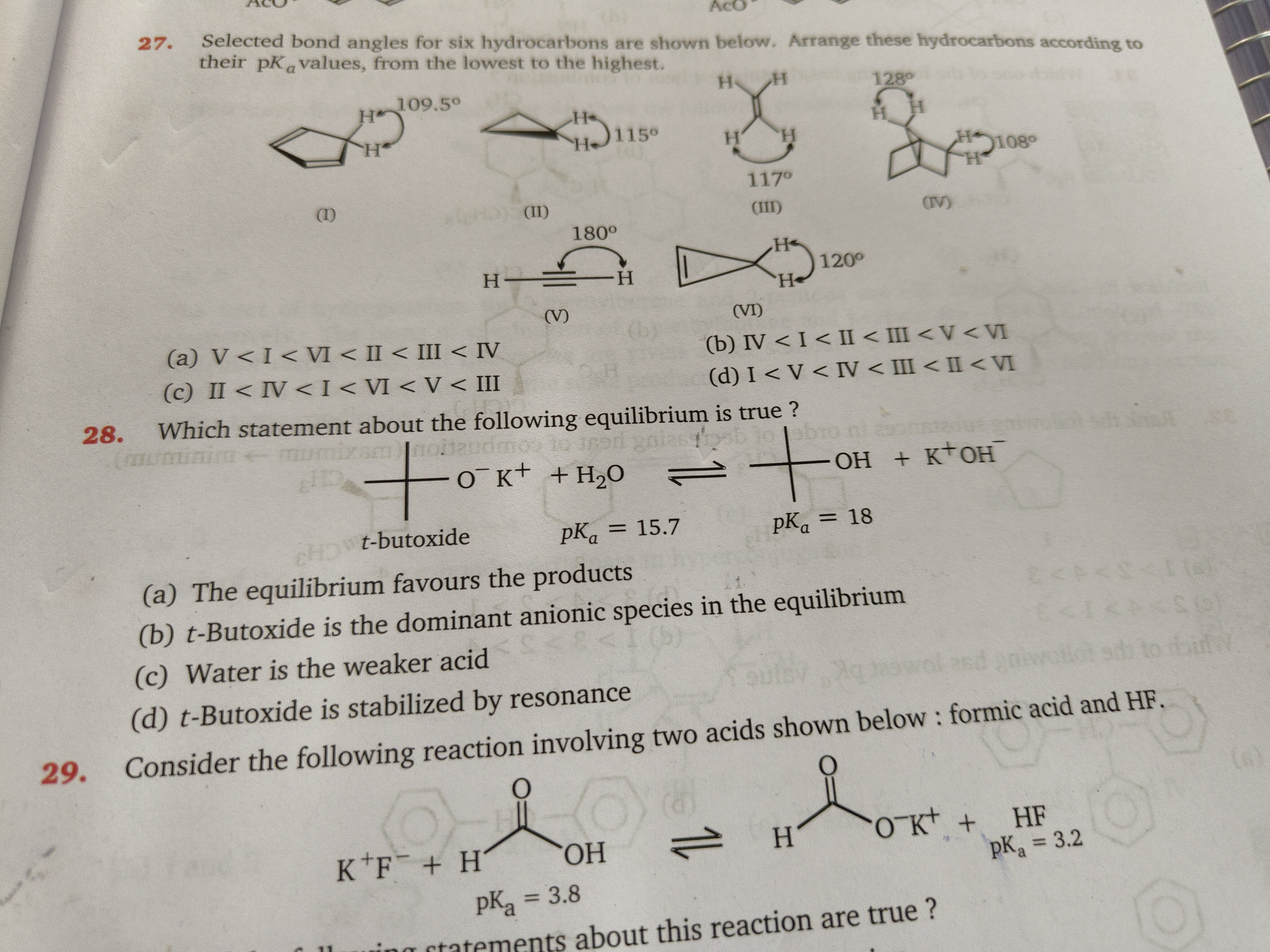
V < I < VI < II < III < IV
IV < I < II < III < V < VI
II < IV < I < VI < V < III
I < V < IV < III < II < VI
No correct option found based on standard chemical principles.
Solution
The acidity of C-H bonds in hydrocarbons is primarily determined by the hybridization of the carbon atom and the stability of the resulting carbanion. The order of acidity based on hybridization is: alkynes (sp) > alkenes (sp²) > alkanes (sp³). Strain in cyclic systems also increases acidity.
- V (Ethyne): sp hybridized C-H bond, pKa≈25.
- III (Ethene): sp² hybridized C-H bond, pKa≈44.
- IV (Norbornane bridgehead): Bridgehead C-H bonds in bicyclic systems like norbornane are more acidic than typical sp³ C-H bonds due to strain and orbital effects, with pKa≈35−38.
- VI (Cyclopropane): sp³ hybridized C-H bond, but high ring strain increases acidity, pKa≈50.
- I (Cyclopentane): sp³ hybridized C-H bond, some ring strain, pKa≈50.
- II (Cyclohexane): sp³ hybridized C-H bond, nearly strain-free, pKa≈50.
Ordering these by acidity (lowest pKa to highest pKa): V (pKa≈25) < IV (pKa≈35−38) < III (pKa≈44) < VI (pKa≈50) < I (pKa≈50) < II (pKa≈50). Among I, II, and VI, cyclopropane (VI) has the highest strain, followed by cyclopentane (I), and then cyclohexane (II). Thus, acidity order is VI > I > II, meaning pKa order is VI < I < II. The complete order is: V < IV < III < VI < I < II. None of the given options match this derived order. Therefore, there appears to be an error in the question or the options provided.
