Question
Question: Calculate the standard enthalpy of formation of benzene from following data. $\Delta_fH^{\circ}(C_6...
Calculate the standard enthalpy of formation of benzene from following data.
ΔfH∘(C6H6)=−3200 kJ,
ΔfH∘(CO2)=−400 kJ mol−1,
ΔfH∘(H2O)=−300 kJ mol−1
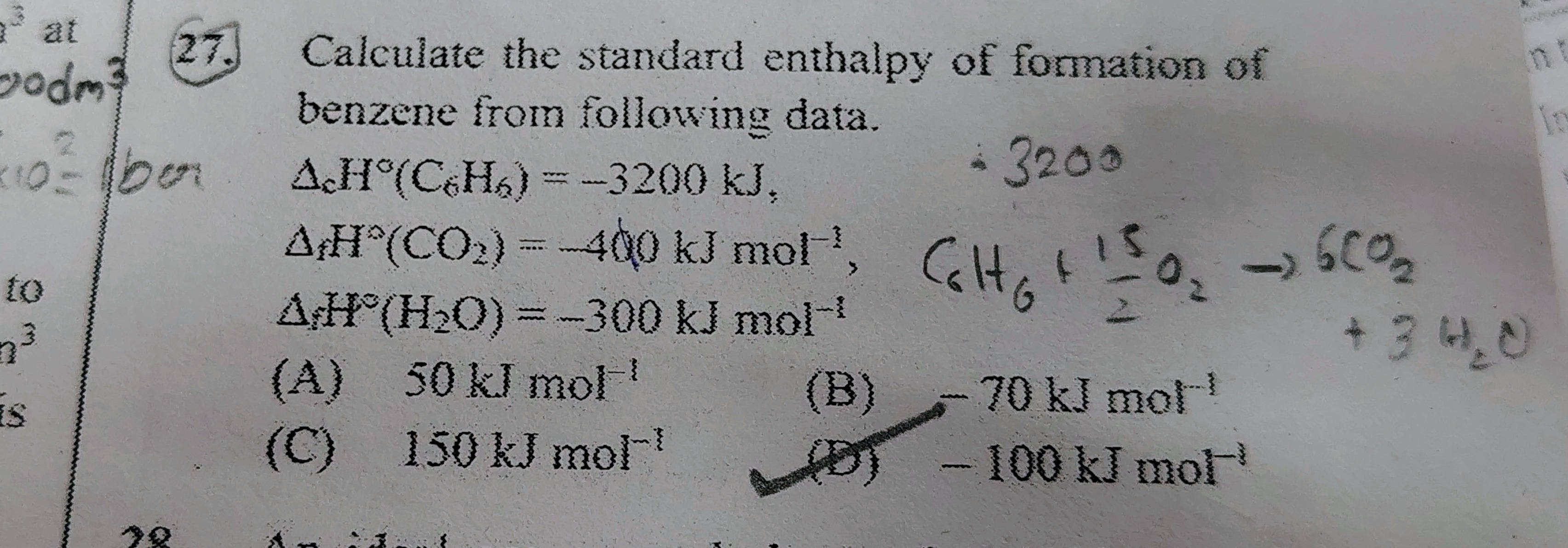
A
50 kJ mol−1
B
-70 kJ mol−1
C
150 kJ mol−1
D
-100 kJ mol−1
Answer
-100 kJ mol−1
Explanation
Solution
For the combustion of benzene:
C6H6+215O2→6CO2+3H2O
Using Hess's law:
ΔcH∘=∑nΔfH∘(products)−ΔfH∘(C6H6)
Given:
ΔcH∘=−3200 kJ,ΔfH∘(CO2)=−400 kJ/mol,ΔfH∘(H2O)=−300 kJ/mol
Calculate the total for products:
6(−400)+3(−300)=−2400−900=−3300 kJ
So,
−3200=−3300−ΔfH∘(C6H6)
Rearrange to solve for ΔfH∘(C6H6):
ΔfH∘(C6H6)=−3300+3200=−100 kJ/mol
