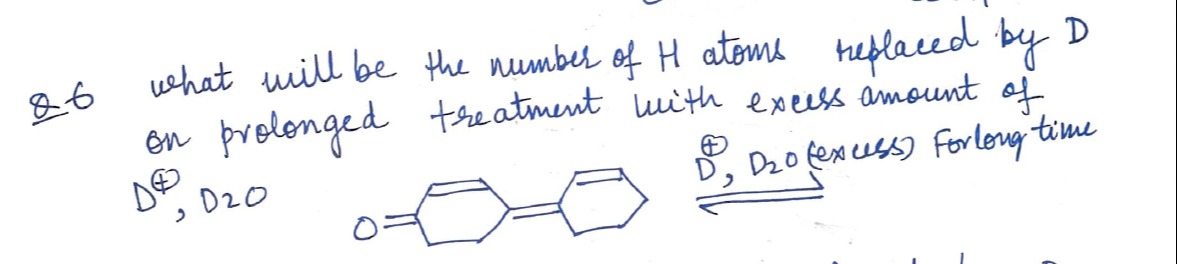
Question
Question: what will be the number of H atoms replaced by D on prolonged treatment with excess amount of $D^{\o...
what will be the number of H atoms replaced by D on prolonged treatment with excess amount of D⊕,D2O
D⊕,D2O(excess) Forlong time
3
Solution
We start by noting that treatment with “D⁺, D₂O (excess)” under acid‐catalysed conditions causes deuterium exchange at sites that can “enolize”. In carbonyl compounds the only “acidic” (i.e. enolizable) protons are those on the α–carbon(s). (The –OH of any transient enol is also rapidly exchanged.)
In the given molecule the two six–membered rings are not fused but connected by a C=C double bond. One ring bears the carbonyl so that it is “enolizable”. Inspection shows that of the two carbons adjacent (α) to the carbonyl group one is “bridging” to the other ring so that it is a methine (–CH–) while the other remains a –CH₂–. Under prolonged condition each of these acidic protons is replaced by deuterium. (No other hydrogen in the molecule, for our conditions, is acidic enough for exchange.)
Thus the number of H–atoms exchanged = 1 (from –CH–) + 2 (from –CH₂–) = 3.
Below is a simplified diagram illustrating the idea:
Explanation (minimal):
- Only the α–hydrogens (adjacent to the C=O) are acidic.
- In this structure one α–carbon (attached to the double bond) is CH (1 H) and the other is CH₂ (2 H).
- Under prolonged deuteration all 3 are replaced.
