Question
Question: 2,6 - Dimethylheptane on monochlorination produces..................derivatives. A) 5 B) 6 C) ...
2,6 - Dimethylheptane on monochlorination produces..................derivatives.
A) 5
B) 6
C) 3
D) 4
Solution
Monochlorination is the addition of chlorine at the terminal methyl groups. The chlorine atom replaces the hydrogen from the methyl group and gets attached to the carbon of the methyl group. Monochlorination means only single chlorine is being added, although multiple chlorine atoms can bind with the molecule based on the availability of methyl groups.
Complete answer:
2,6 - Dimethylheptane is a disubstituted alkane. Two methyl groups are attached to the second and sixth carbon atoms on the chain. The structure of the 2,6 - Dimethylheptane can be drawn as-
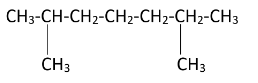
As the diagram suggests, there are four free terminal methyl groups. Monochlorination can occur on all of them. The products formed after mono chlorination will be -
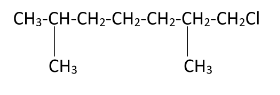
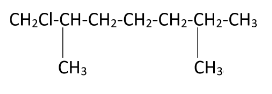
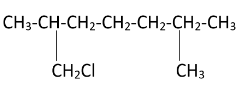
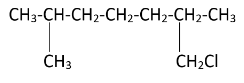
Hence, we can see that there are four possible monochlorination products.
So the correct option is D) 4.
Note:
The products formed can be named as – [7-chloro, 2,6 dimethylheptane], [1-chloro, 2,6 dimethylheptane], [2-chloromethyl, 6-methyl heptane], [6-chloromethyl, 2-methyl heptane]. Simultaneous chlorination can also occur at all the methyl groups present in the molecule resulting in tetrachloro substitution.
