Question
Question: 26. A undergoes a cyclic process ABCA, as shown in the figure. Its pressure at A is P₀. Choose the c...
- A undergoes a cyclic process ABCA, as shown in the figure. Its pressure at A is P₀. Choose the correct options(s) from the following.
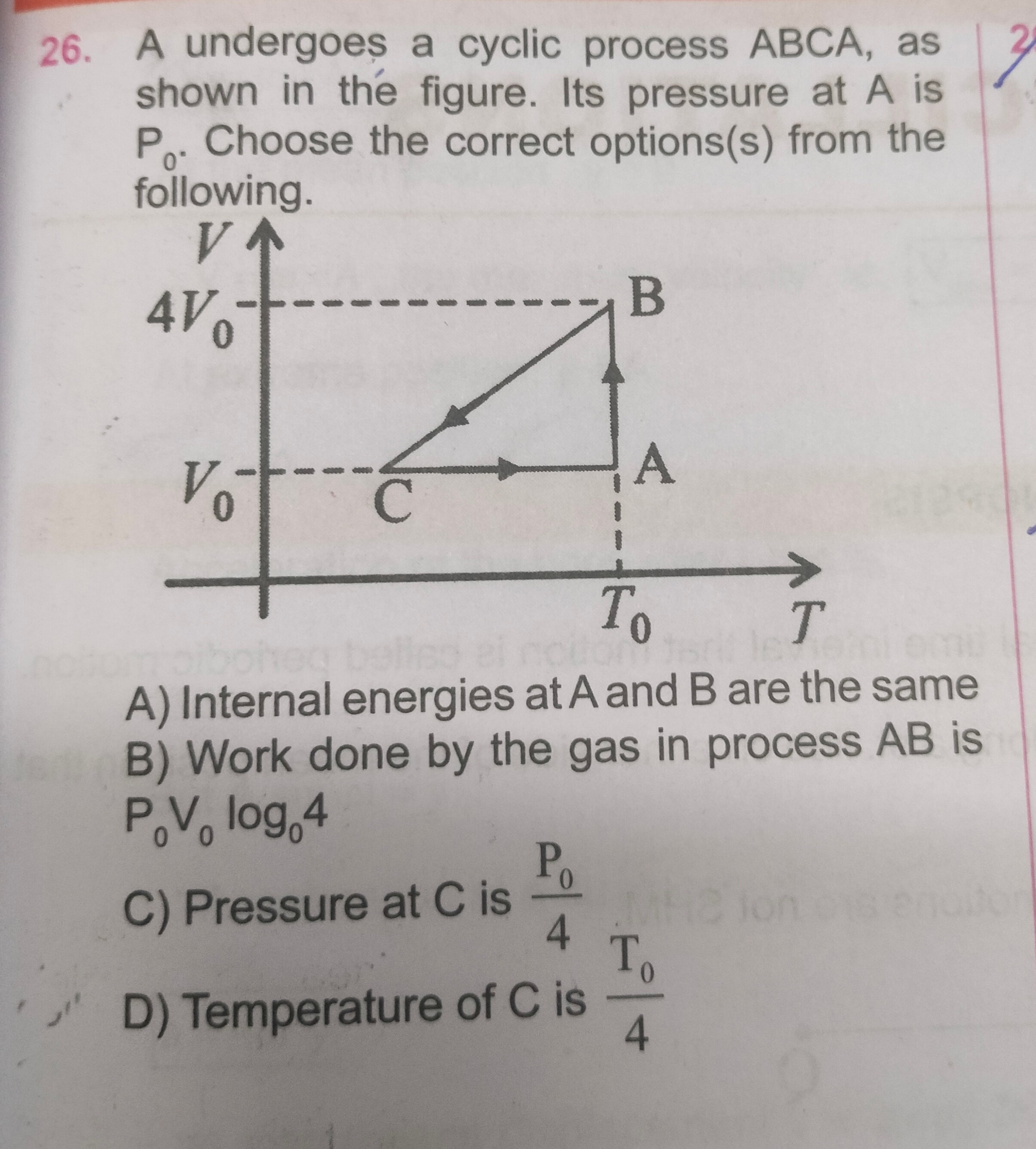
Internal energies at A and B are the same
Work done by the gas in process AB is P₀V₀log₀4
Pressure at C is 4P0
Temperature of C is 4T0
A, B, C, D
Solution
From the V-T diagram:
- Point A: Volume = V0, Temperature = T0. Pressure = P0.
- Point B: Volume = 4V0, Temperature = T0.
- Point C: Volume = V0, Temperature = TC.
Process AB (Isothermal): TA=TB=T0. Using ideal gas law PV=nRT: At A: P0V0=nRT0. At B: PB(4V0)=nRT0. Therefore, P0V0=PB(4V0)⟹PB=4P0.
Process CA (Isochoric): VC=VA=V0. At A: P0V0=nRT0. At C: PCV0=nRTC. Dividing the two equations: P0PC=T0TC⟹PC=P0T0TC.
Process BC (Assuming it passes through origin): V∝T, so TV=constant. From point B: T04V0. From point C: TCV0. Equating: T04V0=TCV0⟹TC=4T0.
Evaluating Options:
A) Internal energies at A and B are the same: Internal energy depends only on temperature for an ideal gas. Since TA=TB=T0, their internal energies are the same. Correct.
B) Work done by the gas in process AB is P0V0loge4: For an isothermal process, W=nRTln(ViVf). WAB=(P0V0)ln(V04V0)=P0V0ln4. Correct.
C) Pressure at C is 4P0: Using PC=P0T0TC and TC=4T0: PC=P0T0T0/4=4P0. Correct.
D) Temperature of C is 4T0: As derived from the assumption that BC passes through the origin. Correct.
