Question
Question: The volume of gases evolved at STP by passing 0.15 A of current for 48.25 min through an aqueous sol...
The volume of gases evolved at STP by passing 0.15 A of current for 48.25 min through an aqueous solution of sodium fumarate is
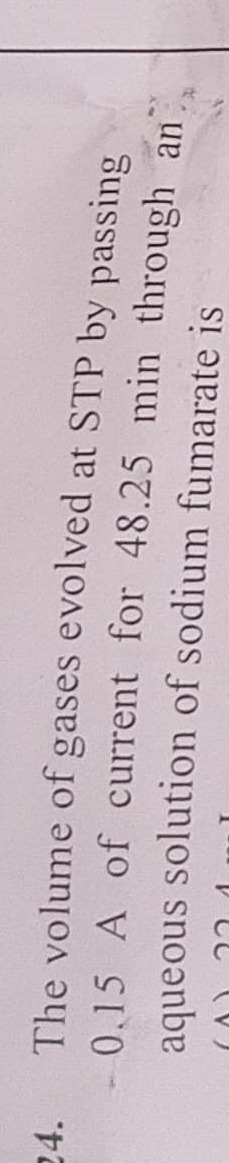
0.202 L
Solution
To determine the volume of gases evolved at STP, we first need to calculate the total charge passed and the moles of electrons involved. Then, we identify the reactions occurring at the anode and cathode and calculate the moles of gases produced.
-
Calculate the total charge (Q) passed: Current (I) = 0.15 A Time (t) = 48.25 min = 48.25 × 60 s = 2895 s Charge (Q) = I × t = 0.15 A × 2895 s = 434.25 C
-
Calculate the moles of electrons (n_e) passed: Faraday's constant (F) = 96485 C/mol Moles of electrons (n_e) = Q / F = 434.25 C / 96485 C/mol = 0.0045006477 mol
-
Identify the reactions at the cathode and anode: The solution contains sodium fumarate (Na2C4H2O4) and water.
-
At the cathode (reduction): Sodium ions (Na+) are harder to reduce than water. Therefore, water will be reduced. 2H2O(l)+2e−→H2(g)+2OH−(aq)
-
At the anode (oxidation): The fumarate ion (C4H2O42−, which is −OOC−CH=CH−COO−) is an organic carboxylate. In the electrolysis of carboxylates (Kolbe electrolysis), the carboxylate groups are oxidized, leading to the formation of CO2 and combination of the remaining hydrocarbon radicals. For fumarate, this yields acetylene (C2H2) and carbon dioxide (CO2). C4H2O42−(aq)→C2H2(g)+2CO2(g)+2e−
-
-
Calculate the moles of each gas produced:
-
From the cathode reaction: For every 2 moles of electrons, 1 mole of H2 is produced. Moles of H2=21×ne=21×0.0045006477 mol=0.00225032385 mol
-
From the anode reaction: For every 2 moles of electrons, 1 mole of C2H2 and 2 moles of CO2 are produced. Moles of C2H2=21×ne=21×0.0045006477 mol=0.00225032385 mol Moles of CO2=22×ne=ne=0.0045006477 mol
-
-
Calculate the total moles of gases evolved: Total moles of gases = Moles of H2 + Moles of C2H2 + Moles of CO2 Total moles of gases = 0.00225032385+0.00225032385+0.0045006477=0.0090012954 mol
-
Calculate the total volume of gases at STP: At STP (Standard Temperature and Pressure), 1 mole of any ideal gas occupies 22.4 L. Volume of gases = Total moles of gases × 22.4 L/mol Volume = 0.0090012954 mol×22.4 L/mol=0.20162901696 L
Rounding to three significant figures, the volume is 0.202 L.
