Question
Question: One mole of an ideal monoatomic gas undergoes expansion along a straight line on P–V curve from init...
One mole of an ideal monoatomic gas undergoes expansion along a straight line on P–V curve from initial state A(3L, 8 atm) to final state B(7.5 L, 2 atm). Calculate q for the above process in L atm
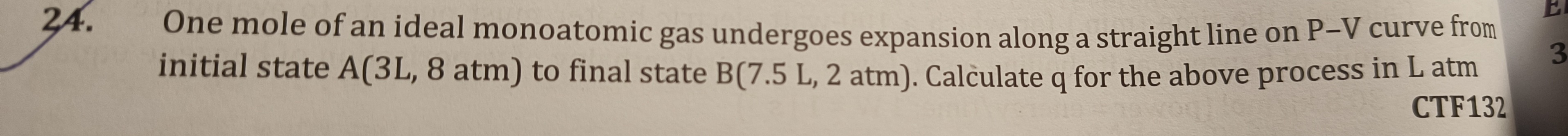
9.0 L atm
Solution
The problem asks to calculate the heat (q) for a process involving one mole of an ideal monoatomic gas expanding along a straight line on a P-V curve.
1. Calculate the work done (w) by the system: The process is a straight line on the P-V curve from state A(3L, 8 atm) to state B(7.5 L, 2 atm). The work done by the system (wby_system) is the area under the P-V curve. This area forms a trapezium. The formula for the area of a trapezium is 21×(sum of parallel sides)×height. Here, the parallel sides are the initial and final pressures (P1=8 atm, P2=2 atm), and the height is the change in volume (V2−V1=7.5 L−3 L).
wby_system=21(P1+P2)(V2−V1) wby_system=21(8 atm+2 atm)(7.5 L−3 L) wby_system=21(10 atm)(4.5 L) wby_system=5×4.5 L atm wby_system=22.5 L atm
2. Calculate the change in internal energy (ΔU): For an ideal gas, the change in internal energy depends only on the change in temperature: ΔU=nCvΔT. For a monoatomic ideal gas, the molar heat capacity at constant volume (Cv) is 23R. So, ΔU=n23RΔT=23(nRT2−nRT1). Using the ideal gas law, PV=nRT, we can substitute nRT with PV: ΔU=23(P2V2−P1V1)
Calculate the initial and final PV products: P1V1=8 atm×3 L=24 L atm P2V2=2 atm×7.5 L=15 L atm
Now, calculate ΔU: ΔU=23(15 L atm−24 L atm) ΔU=23(−9 L atm) ΔU=−13.5 L atm
3. Apply the First Law of Thermodynamics: The First Law of Thermodynamics states that ΔU=q−wby_system (where wby_system is work done by the system). Rearranging to find q: q=ΔU+wby_system
Substitute the calculated values of ΔU and wby_system: q=−13.5 L atm+22.5 L atm q=9.0 L atm
The positive value of q indicates that heat is absorbed by the system during the process.
