Question
Question: Identify the compound in which oxygen exists in the oxidized state?...
Identify the compound in which oxygen exists in the oxidized state?
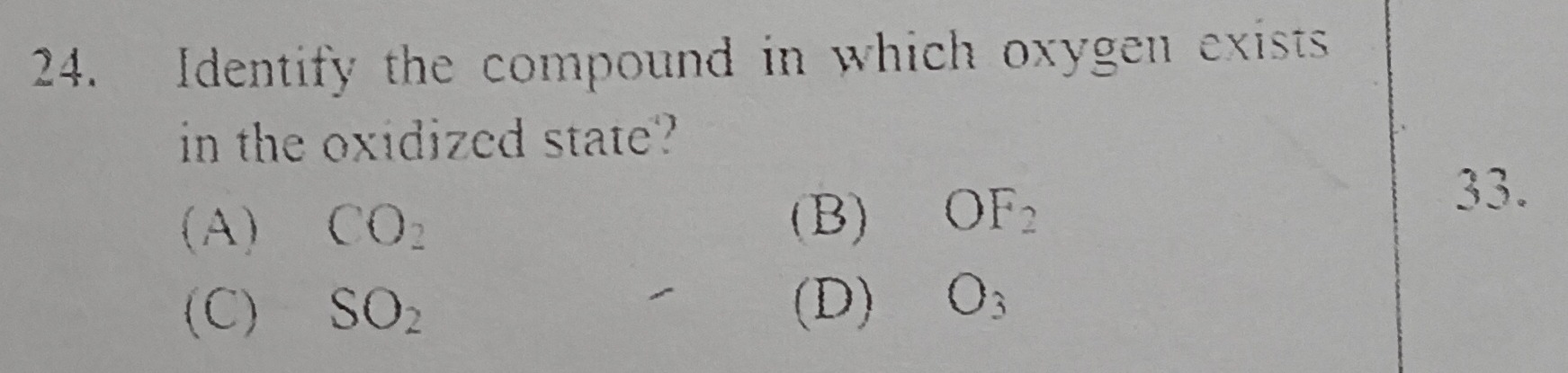
A
CO₂
B
OF₂
C
SO₂
D
O₃
Answer
OF₂
Explanation
Solution
For each compound, determine the oxidation state of oxygen:
- CO₂: Oxygen is -2.
- OF₂: Fluorine is more electronegative, so each F is -1. Thus, for OF₂: let oxygen be x, then x + 2(−1) = 0 ⟹ x = +2.
- SO₂: Oxygen is -2.
- O₃: It’s elemental oxygen, so oxidation state is 0.
Only in OF₂ does oxygen have a positive (oxidized) state.
