Question
Question: Energy level of a hypothetical atom are given as shown. Which transition will give photon of wavelen...
Energy level of a hypothetical atom are given as shown. Which transition will give photon of wavelength 275 nm ?
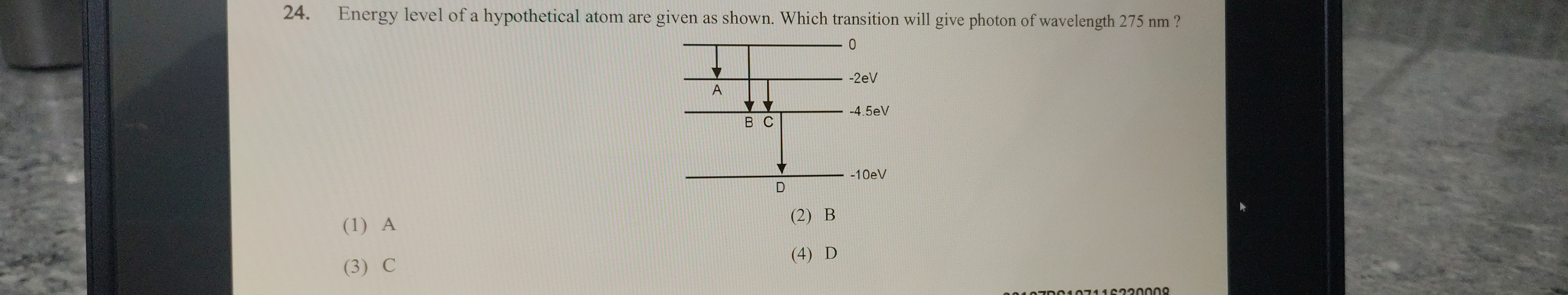
A
B
C
D
B
Solution
-
Calculate Photon Energy: The energy (E) of a photon is related to its wavelength (λ) by the formula:
E=λhc
where h is Planck's constant (6.626×10−34 J s), and c is the speed of light (3×108 m/s). Given λ=275 nm=275×10−9 m.
E=275×10−9 m(6.626×10−34 J s)×(3×108 m/s)≈7.228×10−19 J
-
Convert Energy to Electron Volts (eV): Convert the photon energy from Joules to eV using the conversion factor 1 eV=1.602×10−19 J.
EeV=1.602×10−19 J/eV7.228×10−19 J≈4.51 eV
(Alternatively, use the shortcut E(eV)=λ(nm)1240=2751240≈4.509 eV)
So, the photon has an energy of approximately 4.5 eV.
-
Calculate Energy Differences for Transitions: For each given transition, calculate the energy difference between the initial and final energy levels.
-
Transition A: From 0 eV to −2 eV
ΔEA=0−(−2 eV)=2 eV
-
Transition B: From 0 eV to −4.5 eV
ΔEB=0−(−4.5 eV)=4.5 eV
-
Transition C: From −2 eV to −4.5 eV
ΔEC=−2−(−4.5 eV)=2.5 eV
-
Transition D: From −4.5 eV to −10 eV
ΔED=−4.5−(−10 eV)=5.5 eV
-
-
Match the Energies: Compare the calculated photon energy (4.5 eV) with the energy differences for each transition. Transition B has an energy difference of 4.5 eV, which exactly matches the energy of the photon with a wavelength of 275 nm.
