Question
Question: During a number of complete cycles, a reversible engine (shown by a circle) absorbs 1200 joule from ...
During a number of complete cycles, a reversible engine (shown by a circle) absorbs 1200 joule from reservoir at 400 K and performs 200 joule of mechanical work then:
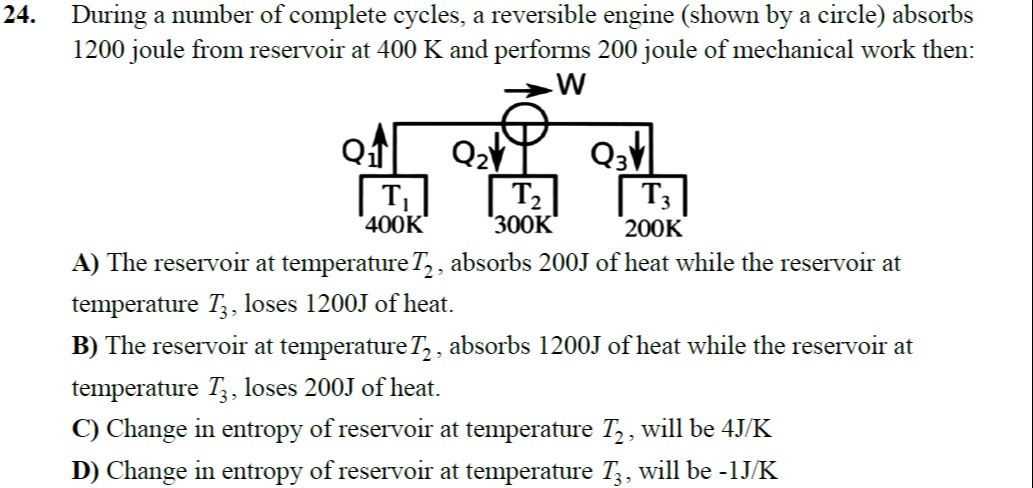
The reservoir at temperature T2, absorbs 200J of heat while the reservoir at temperature T3, loses 1200J of heat.
The reservoir at temperature T2, absorbs 1200J of heat while the reservoir at temperature T3, loses 200J of heat.
Change in entropy of reservoir at temperature T2, will be 4J/K
Change in entropy of reservoir at temperature T3, will be -1J/K
C
Solution
The problem describes a reversible engine operating in a cycle with three heat reservoirs at temperatures T1=400K, T2=300K, and T3=200K. The engine absorbs Q1=1200J from the reservoir at T1=400K and performs work W=200J. Let Q2 be the heat exchanged with the reservoir at T2, and Q3 be the heat exchanged with the reservoir at T3. According to the first law of thermodynamics for a complete cycle, the net heat absorbed equals the net work done:
Q1+Q2+Q3=W 1200+Q2+Q3=200 Q2+Q3=−1000J
(Note: Qi is positive if absorbed by the engine and negative if rejected by the engine).
Since the engine is reversible and operates in a cycle, the total change in entropy of the system (engine + reservoirs) is zero. The change in entropy of the engine over a cycle is zero. The total change in entropy of the reservoirs is zero. The change in entropy of a reservoir at temperature T that exchanges heat Q with the engine is ΔS=−TQ (where Q is the heat absorbed by the engine from the reservoir).
So, for the three reservoirs, the total change in entropy is:
ΔStotal=ΔS1+ΔS2+ΔS3=−T1Q1−T2Q2−T3Q3=0 T1Q1+T2Q2+T3Q3=0 4001200+300Q2+200Q3=0 3+300Q2+200Q3=0 300Q2+200Q3=−3
We have a system of two linear equations:
- Q2+Q3=−1000
- 300Q2+200Q3=−3
From equation (1), Q3=−1000−Q2. Substitute into equation (2): 300Q2+200−1000−Q2=−3 Multiply by 600: 2Q2+3(−1000−Q2)=−3×600 2Q2−3000−3Q2=−1800 −Q2=−1800+3000 −Q2=1200 Q2=−1200J
Now find Q3: Q3=−1000−Q2=−1000−(−1200)=−1000+1200=200J
So, the engine absorbs Q1=1200J from T1=400K, rejects Q2=1200J to T2=300K (since Q2=−1200), and absorbs Q3=200J from T3=200K.
Change in entropy of reservoir at temperature T2 is ΔS2=T2Heat absorbed by reservoir=3001200=4J/K.
