Question
Question: 2,4-Dimethyl-3-pentanol is a: (A) primary alcohol (B) secondary alcohol (C) tertiary alcohol ...
2,4-Dimethyl-3-pentanol is a:
(A) primary alcohol
(B) secondary alcohol
(C) tertiary alcohol
(D) dihydric alcohol
Solution
The structure of 2,4-Dimethyl-3-pentanol is :
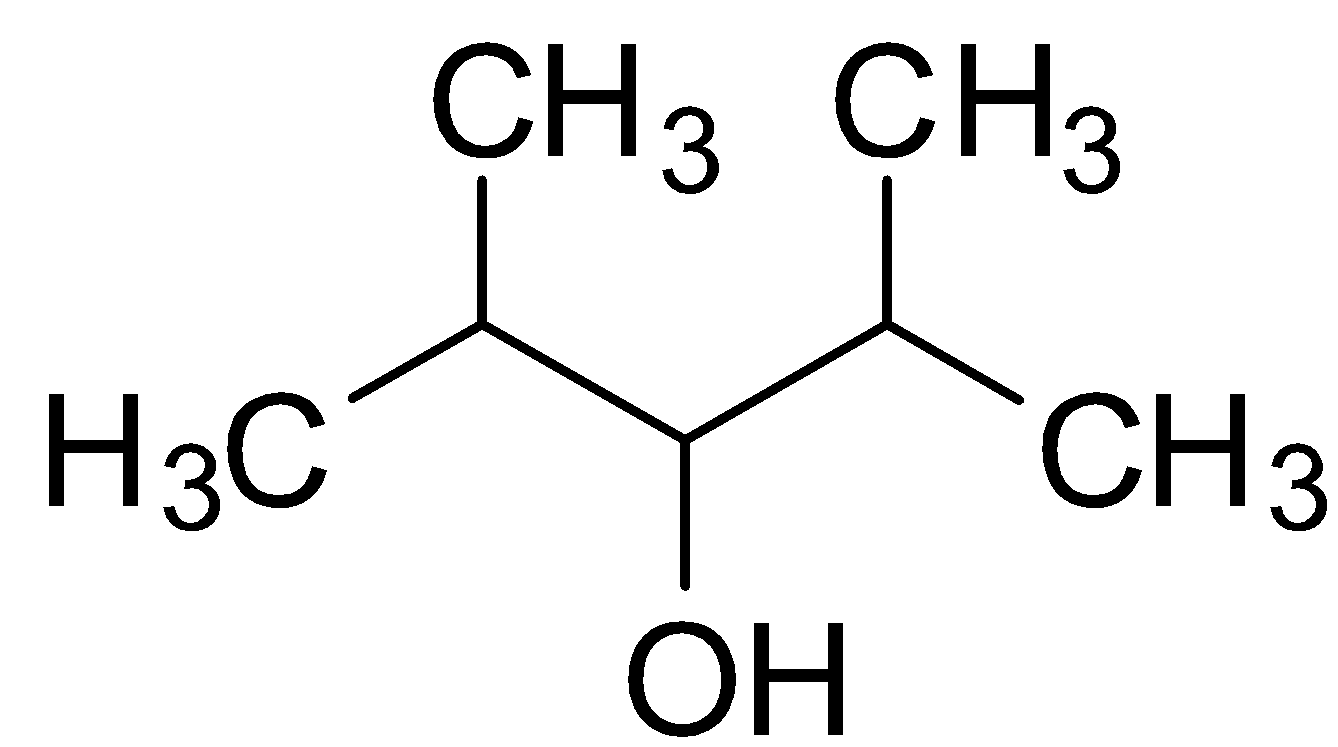
Primary, secondary and tertiary alcohols are classified on the basis of the number of other carbon atoms being attached to the carbon having the hydroxyl (OH) group.
Complete answer:
The classification of alcohols is done on the basis of the attachment of the carbon atom holding the hydroxyl group (OH) to other alkyl groups and is classified as primary, secondary or tertiary alcohols.
In a primary alcohol, the carbon atom that carries the -OH group is only attached to one alkyl group as shown below-
R1−CH2−OH
In a secondary alcohol, the carbon atom with which the -OH group is attached, is further joined directly to two alkyl groups, which may be the same or different.
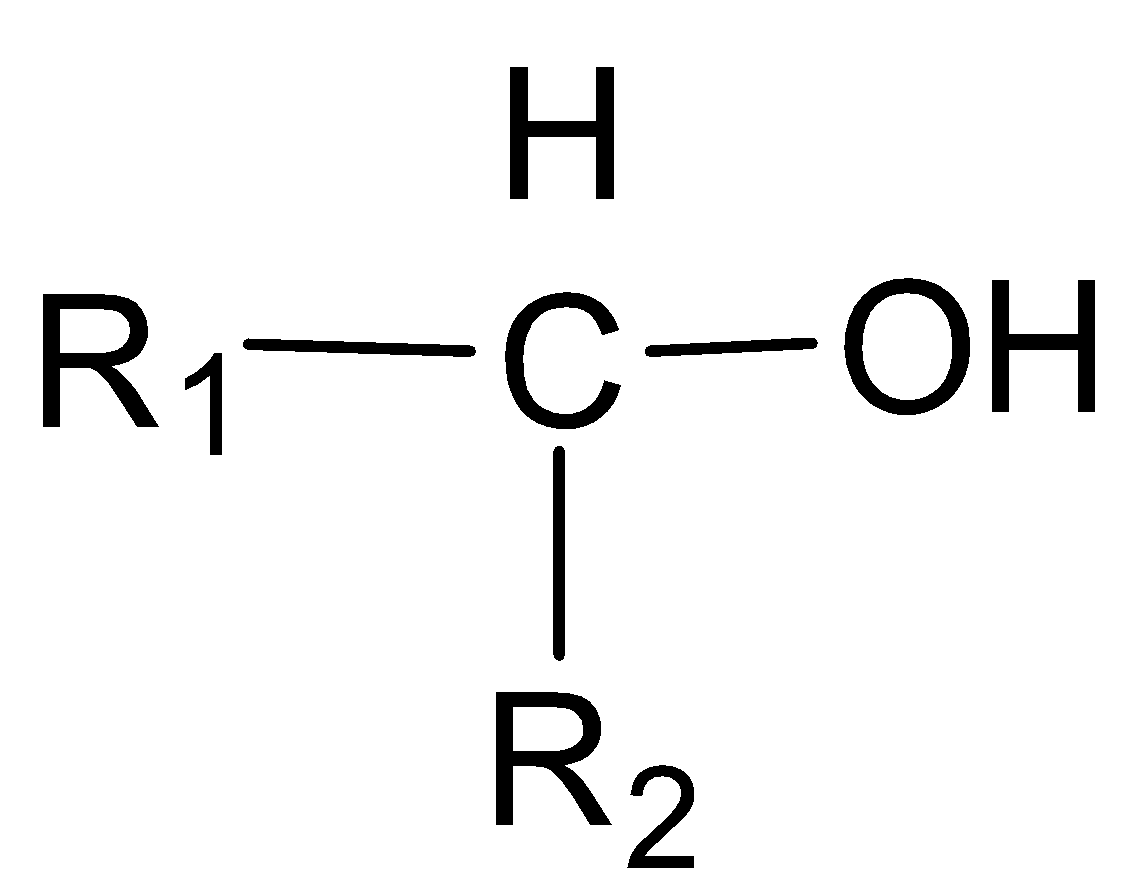 Secondary alcohol
Secondary alcohol
In a tertiary alcohol, the carbon atom with which the -OH group is attached, is further joined directly to three alkyl groups and is not attached to any hydrogen atom.
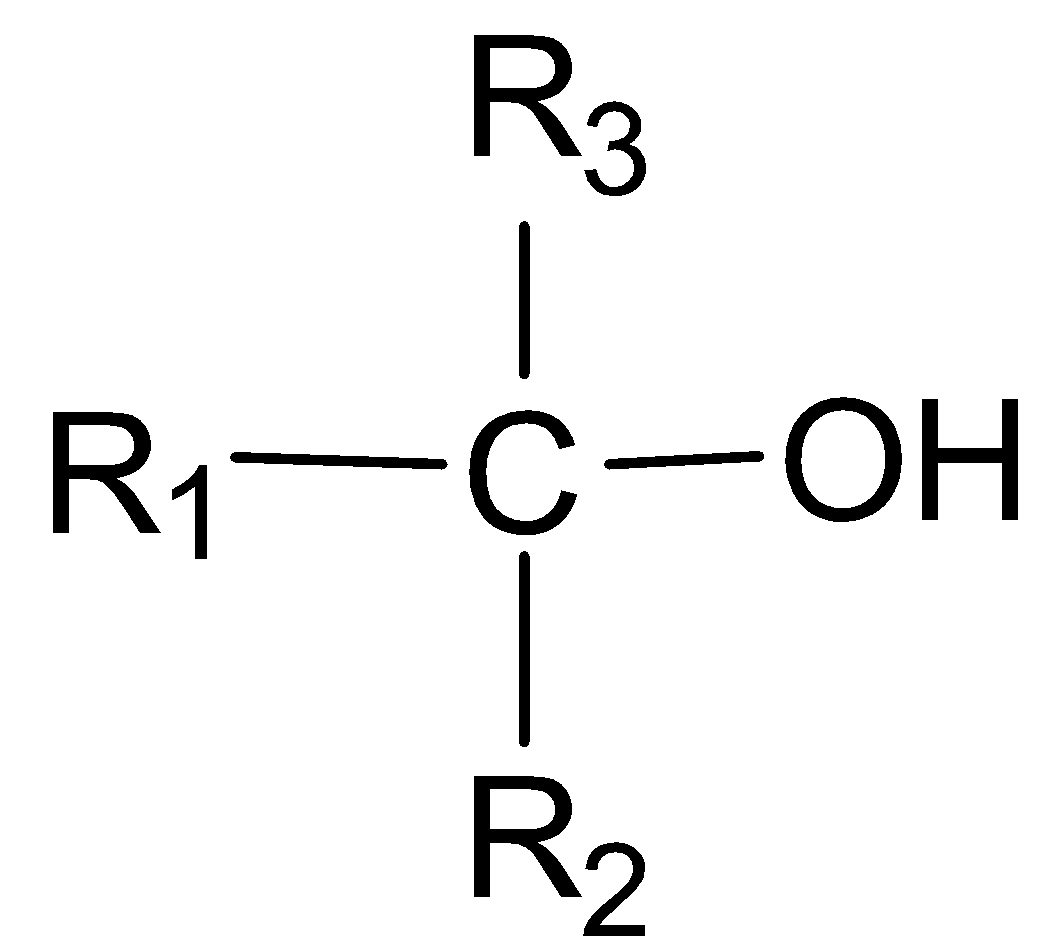 Tertiary alcohol
Tertiary alcohol
The structure of 2,4-Dimethyl-3-pentanol is :
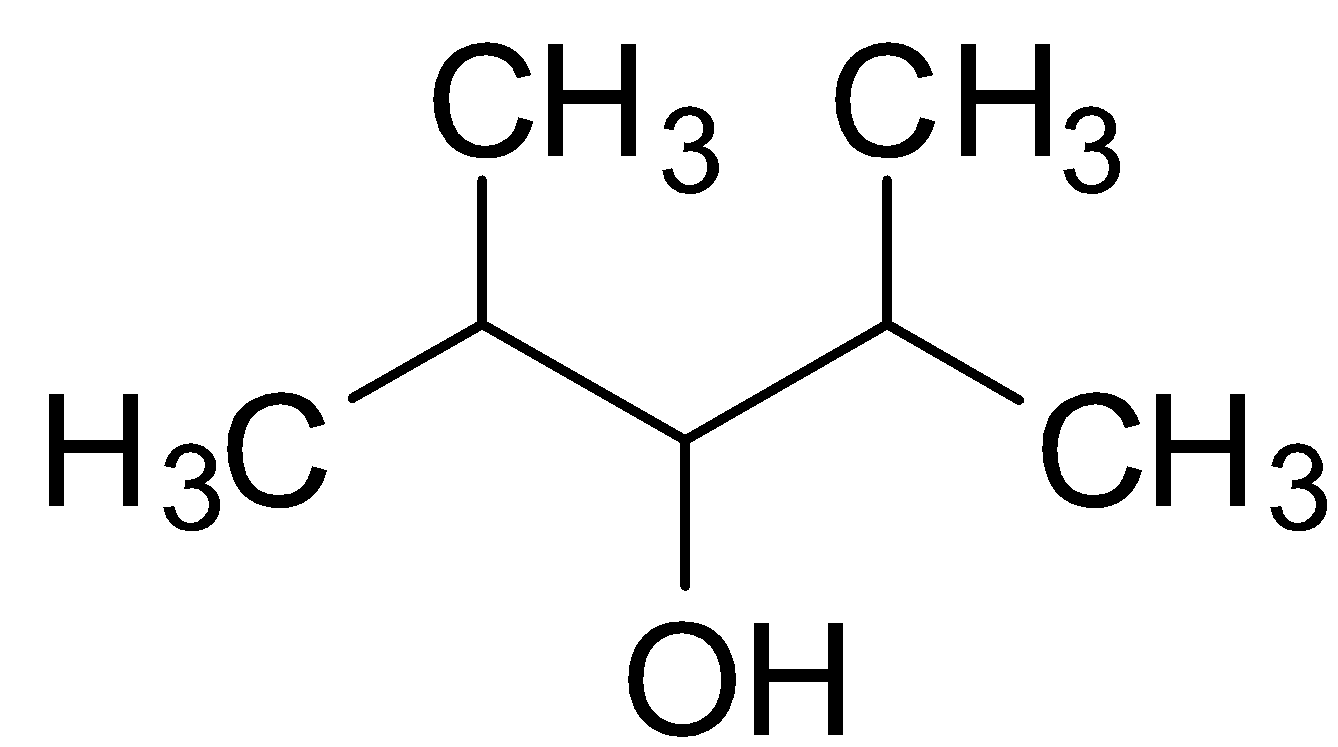
Dihydric alcohols are those classes of alcohols which have 2 hydroxyl (OH) groups in a single molecule. Since 2,4-Dimethyl-3-pentanol has only 1 OH group, it is clearly not a dihydric alcohol. It is rather a monohydric alcohol.
2,4-Dimethyl-3-pentanol is therefore a secondary alcohol because the carbon having OH group is attached to two −CH−(CH3)2 Isopropyl group.
Hence the correct answer is option (B) secondary alcohol.
Note:
In general, primary carbons are carbons attached to one other carbon, secondary carbons attached to two other carbons, tertiary carbons to three other carbons, and quaternary carbons to four other carbons.
