Question
Question: The oxidation number of Tin in $Sn(OH)_6^-$ is...
The oxidation number of Tin in Sn(OH)6− is
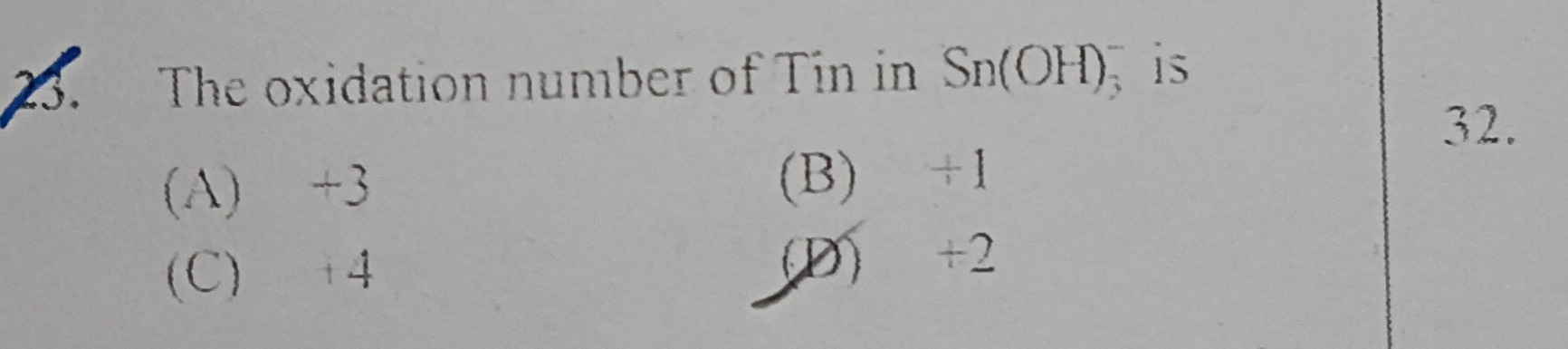
A
+3
B
+1
C
+4
D
+2
Answer
+4
Explanation
Solution
Let the oxidation state of Sn = x.
Each hydroxide (OH⁻) contributes –1. For the complex ion, assuming the common hexahydroxostannate ion is [Sn(OH)6]2−, the total charge balance is:
x+6(–1)=–2
x–6=–2
x=+4
Thus, the oxidation number of tin is +4.
