Question
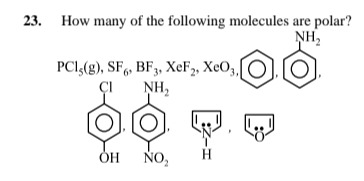
Question: How many of the following molecules are polar? $PCl_5(g), SF_6, BF_3, XeF_2, XeO_3$, , , , , ....
How many of the following molecules are polar? PCl5(g),SF6,BF3,XeF2,XeO3, , , , , .
Answer
6
Explanation
Solution
To determine if a molecule is polar, we need to consider its molecular geometry and the polarity of its individual bonds. A molecule is polar if it has a net dipole moment, which occurs when polar bonds are present and their dipoles do not cancel out due to the molecular geometry.
Let's analyze each molecule:
-
PCl5(g):
- Geometry: Trigonal bipyramidal.
- Bond polarity: P-Cl bonds are polar.
- Symmetry: The molecule is highly symmetrical. The three equatorial P-Cl bond dipoles cancel each other, and the two axial P-Cl bond dipoles cancel each other.
- Net dipole moment: Zero.
- Polarity: Nonpolar.
-
SF6:
- Geometry: Octahedral.
- Bond polarity: S-F bonds are polar.
- Symmetry: The molecule is highly symmetrical. All S-F bond dipoles cancel out.
- Net dipole moment: Zero.
- Polarity: Nonpolar.
-
BF3:
- Geometry: Trigonal planar.
- Bond polarity: B-F bonds are polar.
- Symmetry: The molecule is highly symmetrical. The three B-F bond dipoles cancel out.
- Net dipole moment: Zero.
- Polarity: Nonpolar.
-
XeF2:
- Geometry: Linear (due to 3 lone pairs in equatorial positions and 2 bonding pairs in axial positions).
- Bond polarity: Xe-F bonds are polar.
- Symmetry: The two Xe-F bond dipoles are equal in magnitude and opposite in direction, so they cancel out.
- Net dipole moment: Zero.
- Polarity: Nonpolar.
-
XeO3:
- Geometry: Trigonal pyramidal (due to one lone pair and three Xe=O double bonds).
- Bond polarity: Xe=O bonds are polar.
- Symmetry: The molecule is asymmetrical due to the lone pair and the pyramidal shape. The bond dipoles do not cancel out.
- Net dipole moment: Non-zero.
- Polarity: Polar.
-
Benzene (C6H6):
- Geometry: Planar hexagonal.
- Bond polarity: C-C and C-H bonds are essentially nonpolar or have very small dipoles.
- Symmetry: The molecule is highly symmetrical.
- Net dipole moment: Zero.
- Polarity: Nonpolar.
-
Aniline (C6H5NH2):
- Structure: Benzene ring with an amino (-NH2) group.
- Bond polarity: C-N and N-H bonds are polar. The amino group itself is pyramidal and has a dipole moment.
- Symmetry: The presence of the polar -NH2 group breaks the symmetry of the benzene ring.
- Net dipole moment: Non-zero.
- Polarity: Polar.
-
p-Chlorophenol (C6H4ClOH):
- Structure: Benzene ring with -Cl and -OH groups at para positions.
- Bond polarity: C-Cl, C-O, and O-H bonds are polar. Both -Cl and -OH groups have significant dipole moments.
- Symmetry: Although the groups are at para positions, their individual dipole moments are not equal in magnitude, nor do they perfectly cancel out in a vector sum.
- Net dipole moment: Non-zero.
- Polarity: Polar.
-
p-Nitroaniline (C6H4NH2NO2):
- Structure: Benzene ring with -NH2 and -NO2 groups at para positions.
- Bond polarity: Both -NH2 and -NO2 groups are highly polar. The -NH2 group is electron-donating, and the -NO2 group is strongly electron-withdrawing.
- Symmetry: The dipole moments of these groups largely align along the axis through the para positions, and their effects are additive, leading to a significant net dipole.
- Net dipole moment: Non-zero.
- Polarity: Polar.
-
Pyrrole:
- Structure: Five-membered heterocyclic aromatic ring with a nitrogen atom (N-H).
- Bond polarity: C-N and N-H bonds are polar. The nitrogen atom has a lone pair.
- Symmetry: The molecule is not symmetrical enough for all bond dipoles to cancel.
- Net dipole moment: Non-zero.
- Polarity: Polar.
-
Furan:
- Structure: Five-membered heterocyclic aromatic ring with an oxygen atom.
- Bond polarity: C-O bonds are polar. The oxygen atom has lone pairs.
- Symmetry: The molecule is not symmetrical enough for all bond dipoles to cancel.
- Net dipole moment: Non-zero.
- Polarity: Polar.
Counting the polar molecules:
- XeO3
- Aniline
- p-Chlorophenol
- p-Nitroaniline
- Pyrrole
- Furan
There are 6 polar molecules in the given list.
