Question
Question: For the combustion of one mole acetic acid, work done at 298 K is...
For the combustion of one mole acetic acid, work done at 298 K is
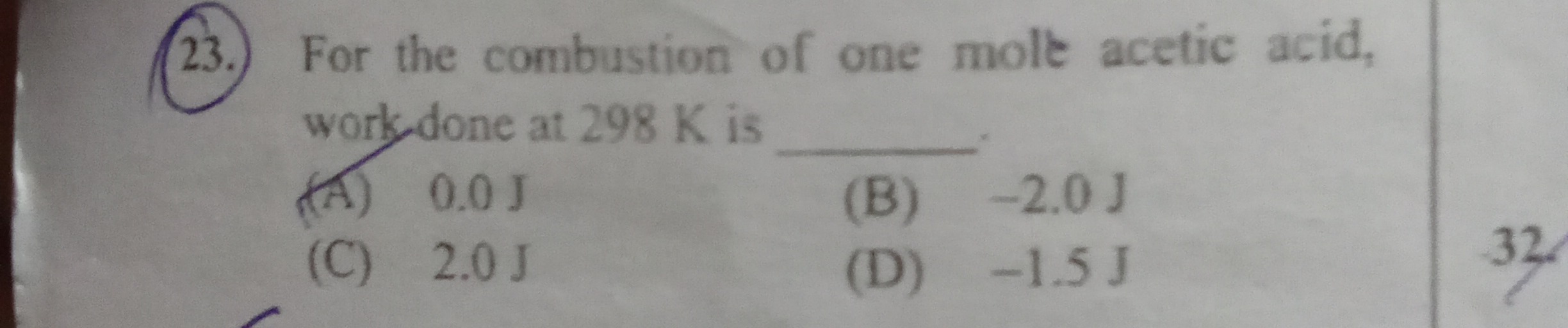
A
0.0 J
B
-2.0 J
C
2.0 J
D
-1.5 J
Answer
0.0 J
Explanation
Solution
The balanced combustion reaction of acetic acid is:
\ceCH3COOH(l)+2O2(g)−>2CO2(g)+2H2O(l)-
Reactant gases: 2 moles (only \ceO2 gas)
-
Product gases: 2 moles (only \ceCO2 gas)
So, the change in moles of gas is:
Δn=2−2=0At constant pressure, the work done is given by:
w=−ΔnRTSince Δn=0, we have:
w=0