Question
Question: Compare carbon-carbon bond rotation across A, B, and C...
Compare carbon-carbon bond rotation across A, B, and C
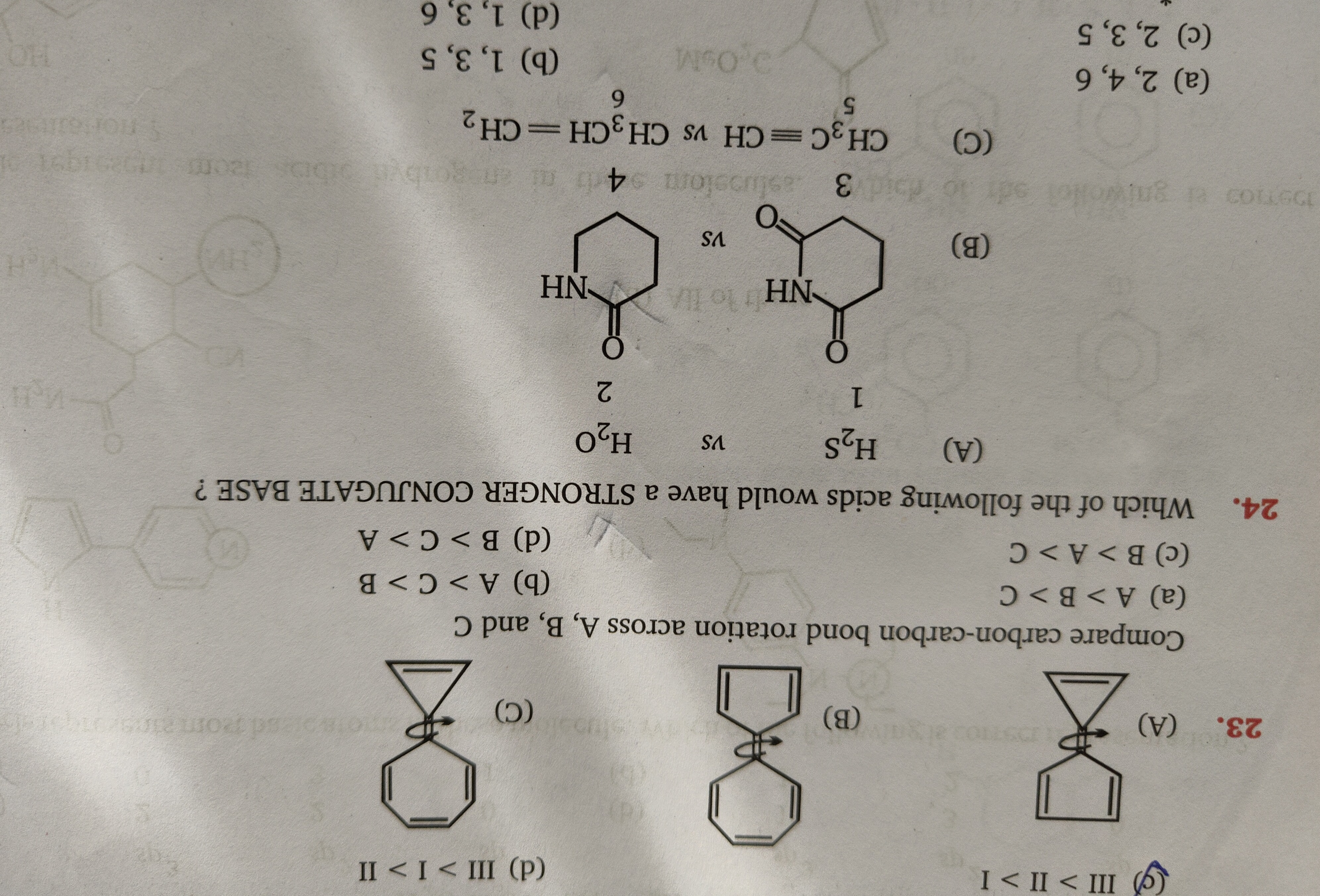
A
A > B > C
B
B > A > C
C
B > C > A
D
A < C < B
Answer
(d) A < C < B
Explanation
Solution
The ease of rotation around a carbon-carbon bond is inversely related to the energy barrier to rotation. This barrier is influenced by ring strain and steric interactions. Structure A (cyclopropene) has the highest ring strain, followed by Structure C (cyclobutene), and then Structure B (cyclopentene), which is relatively strain-free. Higher strain leads to a higher barrier to rotation. Therefore, the ease of rotation is highest for B, followed by C, and then A, which can be represented as A < C < B.
