Question
Question: vander Waal's gas equation may be expressed as $Z=1+\frac{B}{V_m}+\frac{C}{V_m^2}+......$ where Vm=...
vander Waal's gas equation may be expressed as
Z=1+VmB+Vm2C+...... where Vm= molar volume of gas. If B =-0.105Lmol−1 and C=400ml2 mol−2 at 127°C the value of vander Waal constant 'a' (in atm L2mol−2) is (R = 0.08 L-atm/K-mol)
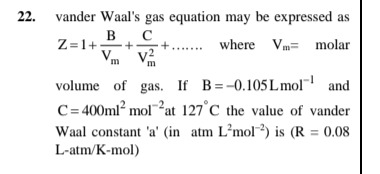
4
Solution
The van der Waals equation for one mole of gas is:
(P+Vm2a)(Vm−b)=RT
To express this in the virial form, we first rearrange it to solve for P:
P=Vm−bRT−Vm2a
Now, we introduce the compressibility factor Z:
Z=RTPVm
Substitute the expression for P into the equation for Z:
Z=RTVm(Vm−bRT−Vm2a)
Z=Vm−bVm−RTVm2aVm
Z=1−b/Vm1−RTVma
We use the binomial expansion for (1−x)−1=1+x+x2+x3+...... where x=b/Vm. Since Vm is typically large for gases, b/Vm is a small quantity.
So, 1−b/Vm1=1+Vmb+Vm2b2+Vm3b3+......
Substitute this back into the expression for Z:
Z=(1+Vmb+Vm2b2+Vm3b3+......)−RTVma
Group terms by powers of 1/Vm:
Z=1+(b−RTa)Vm1+Vm2b2+Vm3b3+......
This is the virial equation of state. Comparing it with the given form:
Z=1+VmB+Vm2C+......
We can identify the second and third virial coefficients:
B=b−RTa
C=b2
Given values:
B=−0.105 L mol−1
C=400 mL2 mol−2
Temperature T=127∘C=127+273=400 K
Gas constant R=0.08 L-atm/K-mol
First, convert the unit of C from mL2 to L2:
1 L=1000 mL
1 mL=10−3 L
C=400 mL2 mol−2=400×(10−3 L)2 mol−2=400×10−6 L2 mol−2=0.0004 L2 mol−2
Now, use the equation for C to find the van der Waals constant 'b':
b2=C
b=C=0.0004 L2 mol−2
b=0.02 L mol−1
Next, use the equation for B to find the van der Waals constant 'a':
B=b−RTa
Rearrange to solve for 'a':
RTa=b−B
a=(b−B)RT
Substitute the values of b, B, R, and T:
a=(0.02 L mol−1−(−0.105 L mol−1))×(0.08 L-atm/K-mol)×(400 K)
a=(0.02+0.105) L mol−1×(0.08×400) L-atm/mol
a=(0.125) L mol−1×(32) L-atm/mol
a=81×32 atm L2 mol−2
a=4 atm L2 mol−2
The value of van der Waals constant 'a' is 4 atm L2 mol−2.
