Question
Question: Identify the compound formed from elements X, Y, Z having oxidation state +2, +5, -2 respectively....
Identify the compound formed from elements X, Y, Z having oxidation state +2, +5, -2 respectively.
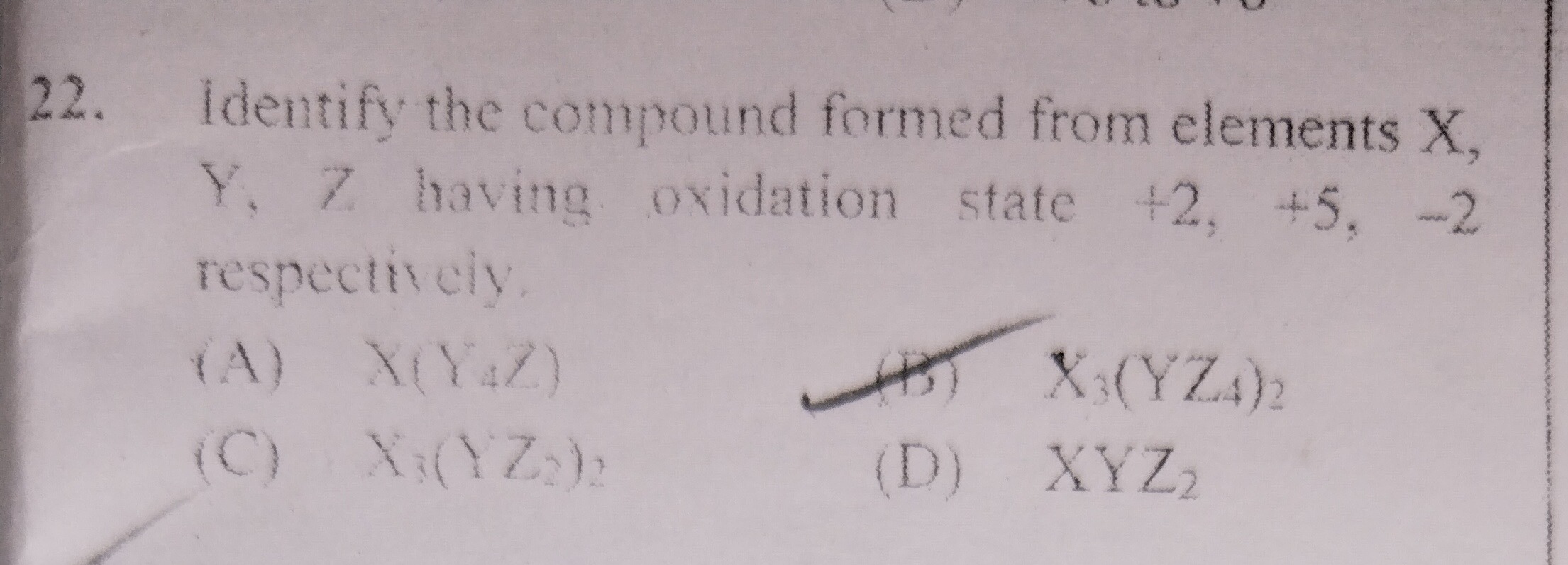
A
X(Y₄Z)
B
X₃(YZ₄)₂
C
X₃(YZ₂)₂
D
XYZ₂
Answer
X₃(YZ₄)₂
Explanation
Solution
Let the compound be Xₘ(YₙZₚ)ₖ.
Given oxidation states:
- X: +2
- Y: +5
- Z: -2
For neutrality, total charge = 0.
Consider option (B): X₃(YZ₄)₂.
- Total charge from X = 3 × (+2) = +6.
- In each (YZ₄) group: charge = (+5) + 4×(–2) = +5 – 8 = –3.
- For two groups: 2 × (–3) = –6.
Sum: +6 + (–6) = 0.
Thus, option (B) is correct.
Balance individual oxidation states: 3(+2) + 2[(+5) + 4(–2)] = 6 + 2(–3) = 0.
