Question
Question: A monoatomic gas is supplied heat 2 Q very slowly keeping the pressure, constant. The work done by t...
A monoatomic gas is supplied heat 2 Q very slowly keeping the pressure, constant. The work done by the gas is
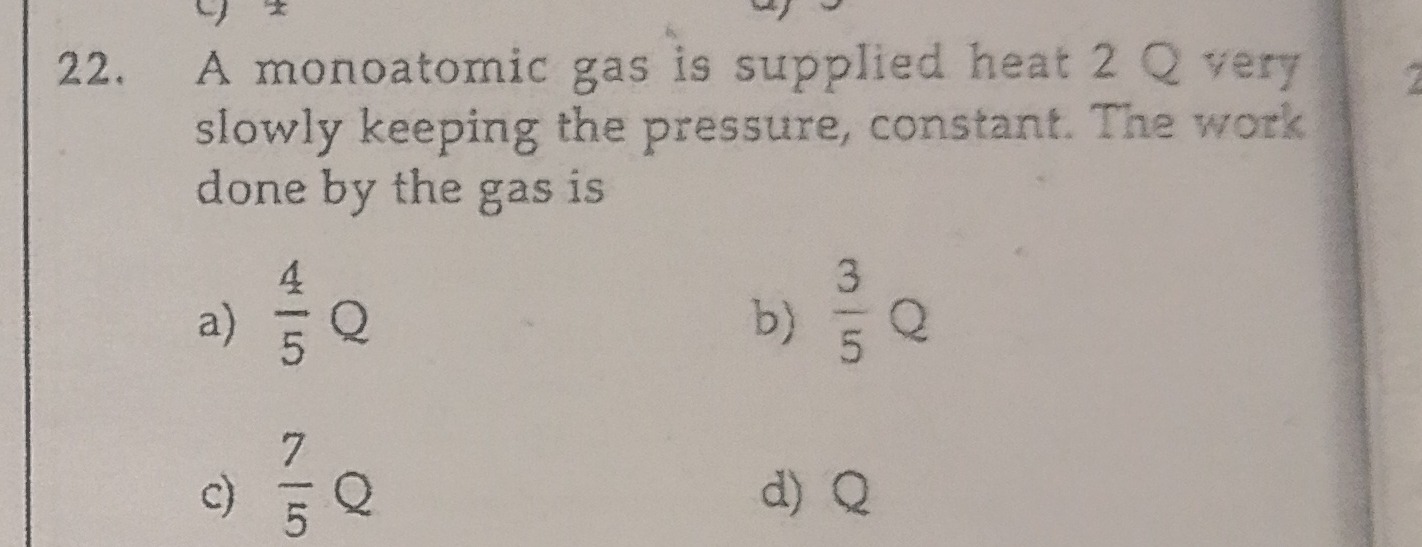
A
54Q
B
53Q
C
57Q
D
Q
Answer
54Q
Explanation
Solution
For a constant pressure process, the heat supplied is given by
nCpΔT=2Q.
For a monoatomic gas,
Cp=25R.
The work done is
W=nRΔT.
Express ΔT from the heat equation:
ΔT=nCp2Q=n25R2Q=5nR4Q.
Thus,
W=nR×5nR4Q=54Q.
