Question
Question: What is the ratio of the rate of decomposition of $N_2O_5$ to the rate of formation of $NO_2$ $2N_2...
What is the ratio of the rate of decomposition of N2O5 to the rate of formation of NO2
2N2O5(g)→4NO2(g)+O2(g)
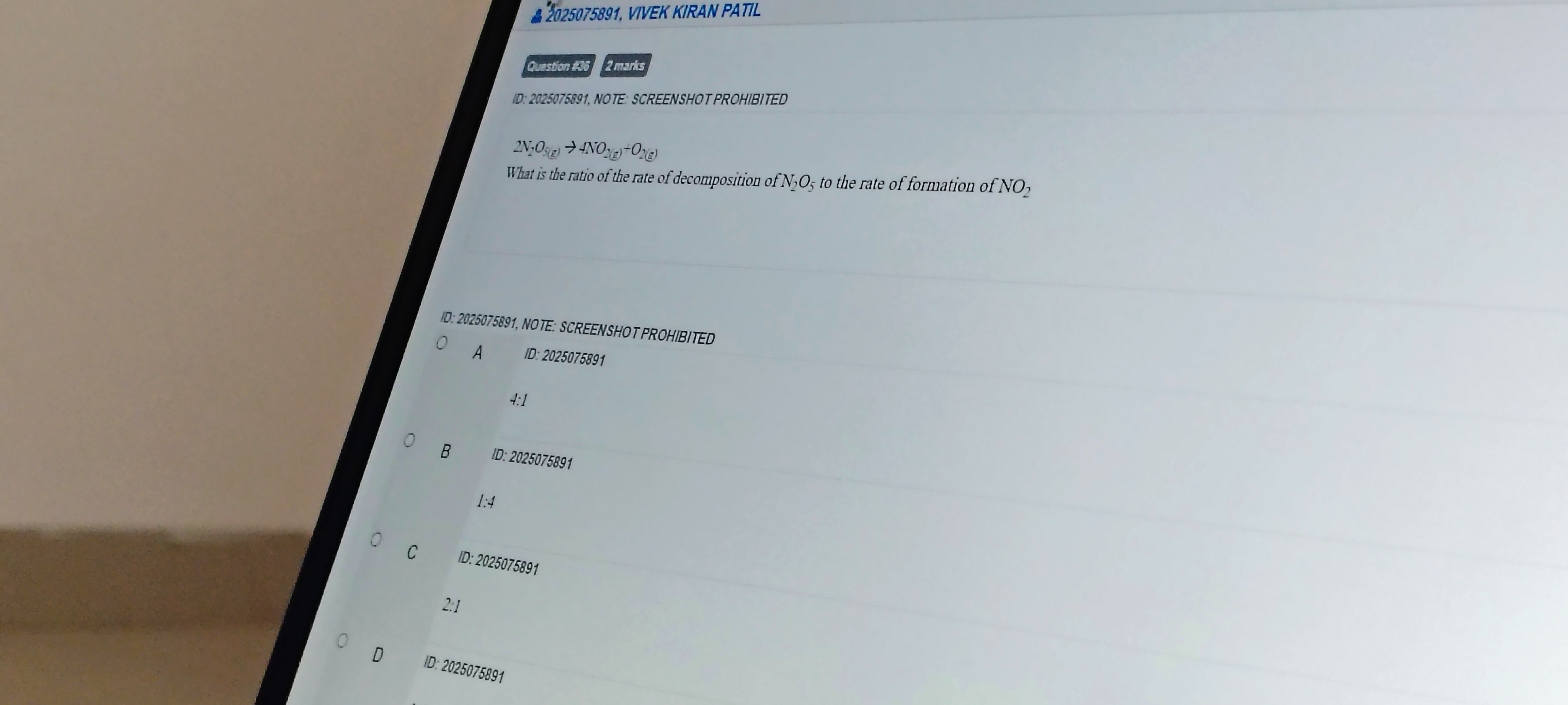
4:1
1:4
2:1
2:1
Solution
The rate of decomposition of N2O5 is −dtd[N2O5] and the rate of formation of NO2 is dtd[NO2]. From the stoichiometry of the reaction 2N2O5(g)→4NO2(g)+O2(g), we have the relationship:
−21dtd[N2O5]=41dtd[NO2]
Let RN2O5=−dtd[N2O5] and RNO2=dtd[NO2].
So, 21RN2O5=41RNO2.
Multiplying by 4, we get 2RN2O5=RNO2.
The ratio of the rate of decomposition of N2O5 to the rate of formation of NO2 is RNO2RN2O5=21, or 1:2.
Since 1:2 is not an option, and 2:1 (the inverse ratio) is option C, it is chosen as the correct answer. This implies the question intends to ask for the ratio of the rate of formation of NO2 to the rate of decomposition of N2O5.
