Question
Question: What is the internal energy change when $X$ J of work is done on the system and $Y$ J of heat is t...
What is the internal energy change when
X J of work is done on the system and
Y J of heat is transferred to surrounding?
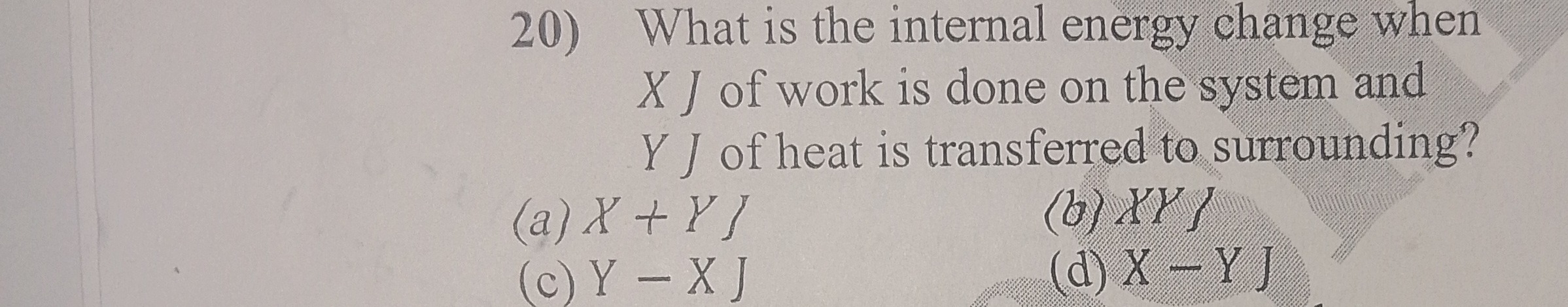
A
X+Y ]
B
XY }
C
Y−X J
D
X−Y J
Answer
X - Y J
Explanation
Solution
Using the first law of thermodynamics:
ΔU=Q−WHere,
- Work done on the system =X J implies work done by the system =−X J.
- Heat transferred to the surroundings means the system loses heat, so Q=−Y J.
Substitute into the equation:
ΔU=(−Y)−(−X)=X−YJ