Question
Question: Identify the major product formed when 2-Methylhexan-3-ol is heated with concentrated sulphuric acid...
Identify the major product formed when 2-Methylhexan-3-ol is heated with concentrated sulphuric acid.
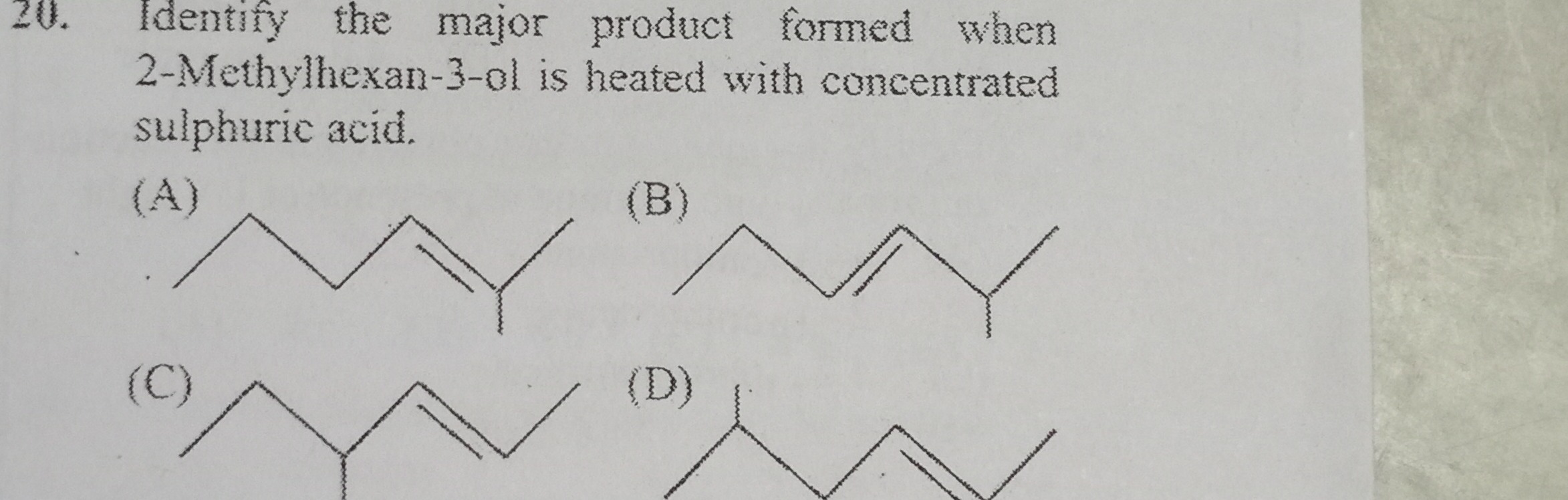
A six-carbon chain with a double bond between carbons 3 and 4. A methyl group is attached to carbon 4.
A six-carbon chain with a double bond between carbons 4 and 5. A methyl group is attached to carbon 5.
A six-carbon chain with a double bond between carbons 2 and 3. A methyl group is attached to carbon 2.
A six-carbon chain with a double bond between carbons 4 and 5. A methyl group is attached to carbon 4.
Option (C)
Solution
The given alcohol is 2‑Methylhexan‑3‑ol. When heated with concentrated H₂SO₄, dehydration occurs via an E1 mechanism. Two possible eliminations are:
- Elimination from C2: Removal of H from C2 leads to a double bond between C2 and C3.
- Elimination from C4: Removal of H from C4 leads to a double bond between C3 and C4.
According to Zaitsev’s rule, the more substituted alkene is favored. Dehydration through removal of a proton from C2 produces an alkene where the double bond is between C2 and C3. C2, being attached to a CH₃ (from C1) and an extra CH₃ (as the substituent on C2), ensures that this double bond is trisubstituted, which is more stable. Option (C) correctly represents the Zaitsev product.
